Pipette-Free and Fully Integrated Paper Device Employing DNA Extraction, Isothermal Amplification, and Carmoisine-Based Colorimetric Detection for Determining Infectious Pathogens
- PMID: 38005500
- PMCID: PMC10675313
- DOI: 10.3390/s23229112
Pipette-Free and Fully Integrated Paper Device Employing DNA Extraction, Isothermal Amplification, and Carmoisine-Based Colorimetric Detection for Determining Infectious Pathogens
Abstract
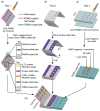
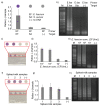
A pipette-free and fully integrated device that can be used to accurately recognize the presence of infectious pathogens is an important and useful tool in point-of-care testing, particularly when aiming to decrease the unpredictable threats posed by disease outbreak. In this study, a paper device is developed to integrate the three main processes required for detecting infectious pathogens, including DNA extraction, loop-mediated isothermal amplification (LAMP), and detection. All key reagents, including sodium dodecyl sulfate (SDS), NaOH, LAMP reagents, and carmoisine, are placed on the paper device. The paper device is operated simply via sliding and folding without using any bulky equipment, and the results can be directly observed by the naked eye. The optimized concentrations of sodium dodecyl sulfate (SDS), sodium hydroxide (NaOH), and carmoisine were found to be 0.1%, 0.1 M, and 0.5 mg/mL, respectively. The paper device was used to detect Enterococcus faecium at concentrations as low as 102 CFU/mL within 60 min. Also, E. faecium spiked in milk was successfully detected using the paper device, demonstrating the feasible application in real sample analysis.
Keywords: Enterococcus faecium; Pipette-free and fully integrated paper device; carmoisine-based colorimetric detection; point-of-care testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Rotatable Paper Device Integrating Reverse Transcription Loop-Mediated Isothermal Amplification and a Food Dye for Colorimetric Detection of Infectious Pathogens.Biosensors (Basel). 2022 Jul 4;12(7):488. doi: 10.3390/bios12070488. Biosensors (Basel). 2022. PMID: 35884291 Free PMC article.
-
D-Glucose-Mediated Gold Nanoparticle Fabrication for Colorimetric Detection of Foodborne Pathogens.Biosensors (Basel). 2024 Jun 1;14(6):284. doi: 10.3390/bios14060284. Biosensors (Basel). 2024. PMID: 38920588 Free PMC article.
-
Fabrication of a fully integrated paper microdevice for point-of-care testing of infectious disease using Safranin O dye coupled with loop-mediated isothermal amplification.Biosens Bioelectron. 2022 May 15;204:114080. doi: 10.1016/j.bios.2022.114080. Epub 2022 Feb 9. Biosens Bioelectron. 2022. PMID: 35176649
-
Copper: DNA extraction and solid phase detection agent for all-in-one molecular diagnostic device coupled with isothermal amplification.Biosens Bioelectron. 2023 Jun 1;229:115222. doi: 10.1016/j.bios.2023.115222. Epub 2023 Mar 11. Biosens Bioelectron. 2023. PMID: 36989581
-
Polydopamine aggregation: A novel strategy for power-free readout of loop-mediated isothermal amplification integrated into a paper device for multiplex pathogens detection.Biosens Bioelectron. 2021 Oct 1;189:113353. doi: 10.1016/j.bios.2021.113353. Epub 2021 May 18. Biosens Bioelectron. 2021. PMID: 34049080
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

