Elevated CO2 Can Improve the Tolerance of Avena sativa to Cope with Zirconium Pollution by Enhancing ROS Homeostasis
- PMID: 38005689
- PMCID: PMC10674191
- DOI: 10.3390/plants12223792
Elevated CO2 Can Improve the Tolerance of Avena sativa to Cope with Zirconium Pollution by Enhancing ROS Homeostasis
Abstract
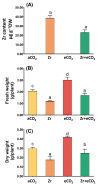
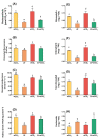
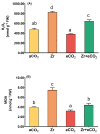
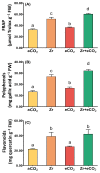
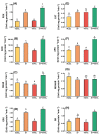
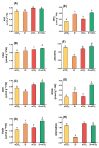
Zirconium (Zr) is one of the toxic metals that are heavily incorporated into the ecosystem due to intensive human activities. Their accumulation in the ecosystem disrupts the food chain, causing undesired alterations. Despite Zr's phytotoxicity, its impact on plant growth and redox status remains unclear, particularly if combined with elevated CO2 (eCO2). Therefore, a greenhouse pot experiment was conducted to test the hypothesis that eCO2 can alleviate the phytotoxic impact of Zr upon oat (Avena sativa) plants by enhancing their growth and redox homeostasis. A complete randomized block experimental design (CRBD) was applied to test our hypothesis. Generally, contamination with Zr strikingly diminished the biomass and photosynthetic efficiency of oat plants. Accordingly, contamination with Zr triggered remarkable oxidative damage in oat plants, with concomitant alteration in the antioxidant defense system of oat plants. Contrarily, elevated levels of CO2 (eCO2) significantly mitigated the adverse effect of Zr upon both fresh and dry weights as well as the photosynthesis of oat plants. The improved photosynthesis consequently quenched the oxidative damage caused by Zr by reducing the levels of both H2O2 and MDA. Moreover, eCO2 augmented the total antioxidant capacity with the concomitant accumulation of molecular antioxidants (e.g., polyphenols, flavonoids). In addition, eCO2 not only improved the activities of antioxidant enzymes such as peroxidase (POX), superoxide dismutase (SOD) and catalase (CAT) but also boosted the ASC/GSH metabolic pool that plays a pivotal role in regulating redox homeostasis in plant cells. In this regard, our research offers a novel perspective by delving into the previously unexplored realm of the alleviative effects of eCO2. It sheds light on how eCO2 distinctively mitigates oxidative stress induced by Zr, achieving this by orchestrating adjustments to the redox balance within oat plants.
Keywords: Avena sativa; antioxidants; ascorbate/glutathione cycle; oxidative damage; toxic metals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yadav S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010;76:167–179. doi: 10.1016/j.sajb.2009.10.007. - DOI
-
- Shanmugaraj B.M., Malla A., Ramalingam S. Cadmium Toxicity and Tolerance in Plants: From Physiology to Remediation. Elsevier Inc.; Amsterdam, The Netherlands: 2018. Cadmium Stress and Toxicity in Plants: An Overview; pp. 1–17.
-
- Goyal D., Yadav A., Prasad M., Singh T.B., Shrivastav P., Ali A., Dantu P.K., Mishra S. Contaminants in Agriculture. Springer International Publishing; Cham, Switzerland: 2020. Effect of Heavy Metals on Plant Growth: An Overview; pp. 79–101.
-
- Perks C., Mudd G. Titanium, Zirconium Resources and Production: A State of the Art Literature Review. Ore Geol. Rev. 2019;107:629–646. doi: 10.1016/j.oregeorev.2019.02.025. - DOI
-
- Song T., Xia C., Ding Y., Liu S., Chen B., Feng Z., Yang T., Li Q. Improvement of Corrosion and Wear Resistance of Novel Zr-Ti-Al-V Alloy with High Strength and Toughness by Thermal Nitridation Treatment. Corros. Sci. 2022;208:110685. doi: 10.1016/j.corsci.2022.110685. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

