Megalurothrips usitatus Directly Causes the Black-Heads and Black-Tail Symptoms of Cowpea along with the Production of Insect-Resistance Flavonoids
- PMID: 38005760
- PMCID: PMC10675644
- DOI: 10.3390/plants12223865
Megalurothrips usitatus Directly Causes the Black-Heads and Black-Tail Symptoms of Cowpea along with the Production of Insect-Resistance Flavonoids
Abstract
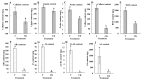
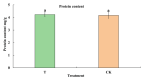
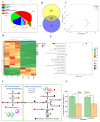
The thrip (Megalurothrips usitatus) damages the flowers and pods of the cowpea, causing "black-heads and black-tails" (BHBT) symptoms and negatively affecting its economic value. However, the mechanism by which BHBT symptoms develop is still unknown. Our results showed that the microstructure of the pod epidermis was altered and the content of the plant's resistance-related compounds increased after a thrip infestation. However, the contents of protein and free amino acids did not change significantly, suggesting that the nutritional value was not altered. Pathogens were found not to be involved in the formation of BHBT symptoms, as fungi and pathogenic bacteria were not enriched in damaged pods. Two herbivory-induced flavonoids-7,4'-dihydroxyflavone and coumestrol-were found to exert insecticidal activity. Our study clarified that BHBT symptoms are directly caused by the thrip. Thresholds for pest control need to be reconsidered as thrip herbivory did not degrade cowpea nutrition.
Keywords: Megalurothrips usitatus; cowpea; flavonoid; nutrition; pathogen; thrip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A Maximum Dose Bioassay to Assess Efficacy of Spinetoram against Cowpea Thrip Megalurothrips usitatus in China.Insects. 2024 Jun 3;15(6):412. doi: 10.3390/insects15060412. Insects. 2024. PMID: 38921127 Free PMC article.
-
Defensive Resistance of Cowpea Vigna unguiculata Control Megalurothrips usitatus Mediated by Jasmonic Acid or Insect Damage.Plants (Basel). 2023 Feb 19;12(4):942. doi: 10.3390/plants12040942. Plants (Basel). 2023. PMID: 36840292 Free PMC article.
-
Megalurothrips usitatus Feeding-Induced De Novo Synthesis of the C8 Volatiles 1-Octen-3-ol and 2-Ethyl-1-hexanol in Cowpea Plants Regulates Plant-Herbivore-Predator Interactions.J Agric Food Chem. 2025 Apr 16;73(15):8842-8851. doi: 10.1021/acs.jafc.4c13266. Epub 2025 Apr 5. J Agric Food Chem. 2025. PMID: 40186560
-
Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges.Plants (Basel). 2022 Jun 15;11(12):1583. doi: 10.3390/plants11121583. Plants (Basel). 2022. PMID: 35736733 Free PMC article. Review.
-
Total polyphenols and bioactivity of seeds and sprouts in several legumes.Curr Pharm Des. 2013;19(34):6112-24. doi: 10.2174/1381612811319340005. Curr Pharm Des. 2013. PMID: 23448441 Review.
Cited by
-
Field-Based Evaluation of Insecticide Effectiveness on Megalurothrips usitatus in Guangdong, China: Implications for Pest Control Strategies.Insects. 2025 Apr 27;16(5):459. doi: 10.3390/insects16050459. Insects. 2025. PMID: 40429172 Free PMC article.
-
Overexpression of the Glycyrrhiza uralensis Phenylalanine Ammonia-Lyase Gene GuPAL1 Promotes Flavonoid Accumulation in Arabidopsis thaliana.Int J Mol Sci. 2025 Apr 25;26(9):4073. doi: 10.3390/ijms26094073. Int J Mol Sci. 2025. PMID: 40362312 Free PMC article.
References
-
- Muhammad A., Malgwi A.M., Nahunnaro H., Adamu R.S., Tamò M., Dannon E., Datinon B. Effect of sowing dates, intra-row spacings and pesticides on cowpea pod borer, Maruca vitrata (Fab.) [Lepidoptera: Crambidae] populations on cowpea, Vigna unguiculata L. (Walp) in Katsina, Sudan savanna. Int. J. Trop. Insect Sci. 2022;42:581–589. doi: 10.1007/s42690-021-00576-7. - DOI
-
- Gerrano A.S., Thungo Z.G., Shimelis H., Mashilo J., Mathew I. Genotype-by-environment interaction for the contents of micro-nutrients and Protein in the green pods of cowpea (Vigna unguiculata L. Walp.) Agriculture. 2022;12:531. doi: 10.3390/agriculture12040531. - DOI
-
- MacWilliams J.R., Chesnais Q., Nabity P., Mauck K., Kaloshian I. Cowpea aphid resistance in cowpea line CB77 functions primarily through antibiosis and eliminates phytotoxic symptoms of aphid feeding. J. Pest Sci. 2023;96:539–553. doi: 10.1007/s10340-022-01529-w. - DOI
-
- Fotso T.G., Tofel H.K., Abdou J.P., Tchao N., Zourmba C.M., Adler C., Nukenine E.N. Control of Callosobruchus maculatus (Coleoptera: Chrysomelidae) using fractionated extracts from cameroonian Hemizygia welwitschii (Lamiaceae) leaf on stored Vigna unguiculata (Fabales: Fabaceae) J. Insect Sci. 2019;19:22. doi: 10.1093/jisesa/iez029. - DOI - PMC - PubMed
Grants and funding
- ZDYF2021XDNY302/Hainan Province Science and Technology Special Fund
- SCKJ-JYRC-2022-72/the Project of Sanya Yazhou Bay Science and Technology City
- 323RC521/Hainan Provincial Natural Science Foundation of China
- HSPHDSRF-2022-4-066/Hainan Special Ph.D Scientific Research Foundation of Sanya Yazhou Bay Science and Technology City
- B21HJ0401/Hainan Yazhou Bay Seed Laboratory
LinkOut - more resources
Full Text Sources

