Optimization and Characterization of Phenolic Extraction Conditions and Antioxidant Activity Evaluation of Adenanthera pavonina L. Bark
- PMID: 38005799
- PMCID: PMC10674903
- DOI: 10.3390/plants12223902
Optimization and Characterization of Phenolic Extraction Conditions and Antioxidant Activity Evaluation of Adenanthera pavonina L. Bark
Abstract
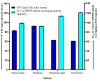
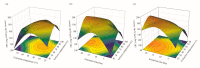
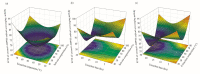
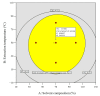
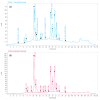
The presence of high levels of secondary metabolites in medicinal plants can significantly influence the progress of drug development. Here, we aimed to maximize phenolic extraction from Adenanthera pavonina L. stem bark using various solvents such as ethyl acetate, methanol, petroleum ether, and chloroform. A response surface method (RSM) with a central composite design (CCD) statistical technique was applied to optimize the extraction process, employing three important extracting parameters such as extraction time (h), temperature (°C), and solvent composition (% v/v of methanol/water) to obtain the highest phenolic content. Total phenolic content (TPC) and antioxidant activity (IC50 of extract's DPPH radical scavenging activity) were used as response variables to find the influence of these extracting parameters. Among the various solvents used, methanol extract showed the highest contents of phenolics and the maximum level of antioxidant activity with a lower IC50 value. The notable TPC and IC50 value of the extract's DPPH radical scavenging capacity were found to be 181.69 ± 0.20 mg GAE/g dry tissue and 60.13 ± 0.11 mg/mL, respectively, under the optimal conditions with a solvent composition of 71.61% (v/v) of methanol/water, extraction temperature of 42.52 °C, and extraction time of 24 h. The optimized extract of A. pavonina stem bark was further subjected to HPLC analysis, where six phenolic compounds, including coumarin, p-coumaric acid, chlorogenic acid, sinapic acid, gallic acid, and caffeic acid, were identified along with their respective quantities. Overall, the findings of this study uncover a low-cost analytical model for maximizing phenolic extraction from A. pavonina bark with enhanced antioxidant activity.
Keywords: Adenanthera pavonina bark; antioxidant activity; high-performance liquid chromatography; optimization; phenolic profiling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds.BMC Complement Altern Med. 2014 Feb 4;14:45. doi: 10.1186/1472-6882-14-45. BMC Complement Altern Med. 2014. PMID: 24495381 Free PMC article.
-
In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina.BMC Complement Altern Med. 2016 Nov 15;16(1):466. doi: 10.1186/s12906-016-1452-y. BMC Complement Altern Med. 2016. PMID: 27846876 Free PMC article.
-
Ultrasound-Assisted Aqueous Extraction of Phenolic, Flavonoid Compounds and Antioxidant Activity of Mucuna macrocarpa Beans: Response Surface Methodology Optimization.J Am Coll Nutr. 2019 May-Jun;38(4):364-372. doi: 10.1080/07315724.2018.1524315. Epub 2018 Dec 27. J Am Coll Nutr. 2019. PMID: 30589617
-
Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts.PeerJ. 2018 Aug 3;6:e5331. doi: 10.7717/peerj.5331. eCollection 2018. PeerJ. 2018. PMID: 30083463 Free PMC article.
-
Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers' Spent Grain Using Response Surface Methodology (RSM).Foods. 2020 Oct 2;9(10):1398. doi: 10.3390/foods9101398. Foods. 2020. PMID: 33023120 Free PMC article.
Cited by
-
Antioxidant Properties of Lippia alba Essential Oil: A Potential Treatment for Oxidative Stress-Related Conditions in Plants and Cancer Cells.Int J Mol Sci. 2024 Jul 29;25(15):8276. doi: 10.3390/ijms25158276. Int J Mol Sci. 2024. PMID: 39125846 Free PMC article.
-
Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology.Plants (Basel). 2024 Sep 12;13(18):2560. doi: 10.3390/plants13182560. Plants (Basel). 2024. PMID: 39339535 Free PMC article.
-
Exploring the Epicarp Potential from Acrocomia aculeata Fruits: Chemical Analysis, Antioxidant and Antimicrobial Activities.Antioxidants (Basel). 2025 Feb 4;14(2):181. doi: 10.3390/antiox14020181. Antioxidants (Basel). 2025. PMID: 40002368 Free PMC article.
-
Study on optimization of extraction and purification processes for total flavonoids from Lycopi herba roots and their anti-proliferative effects on fibrous synoviocytes in human rheumatoid arthritis.Ultrason Sonochem. 2025 Jan;112:107164. doi: 10.1016/j.ultsonch.2024.107164. Epub 2024 Nov 19. Ultrason Sonochem. 2025. PMID: 39579583 Free PMC article.
References
-
- Kirtikar K.R., Basu B.D. Indian Medicinal Plants. 2nd ed. Lalit Mohan Basu; Allahabad, India: 1991. pp. 909–910.
-
- Gerona M.S., Melo R.C., Barros H.L.M., Aquino S.R., Islam M.T., dos Santos Rizzo M., da Costa M.P. Advances in the research of Adenanthera pavonina: From traditional use to intellectual property. J. Med. Plant Res. 2020;14:24–53. doi: 10.5897/JMPR2019.6872. - DOI
-
- Partha G., Rahaman C.H. Pharmacognostic, phytochemical and antioxidant studies of Adenantherapavonina L. Int. J. Pharmacogn. Phytochem. Res. 2015;7:30–37.
-
- Dash S., Das C., Sahoo D.C. Phytochemical and anthelmintic screening of crude bark extract of Adenanthera pavonina Linn. Pharm. Glob. Int. J. Compr. Pharm. 2010;1:1–4.
-
- Ara A., Arifuzzaman M., Ghosh C.K., Hashem M.A., Ahmad M.U., Bachar S.C., Nahar L., Sarker S.D. Anti-inflammatory activity of Adenanthera pavonina L., Fabaceae, in experimental animals. Rev. Bras. Farmacogn. 2010;20:929–932. doi: 10.1590/S0102-695X2010005000039. - DOI
LinkOut - more resources
Full Text Sources

