A Method to Generate and Rescue Recombinant Adenovirus Devoid of Replication-Competent Particles in Animal-Origin-Free Culture Medium
- PMID: 38005830
- PMCID: PMC10674172
- DOI: 10.3390/v15112152
A Method to Generate and Rescue Recombinant Adenovirus Devoid of Replication-Competent Particles in Animal-Origin-Free Culture Medium
Abstract
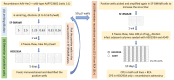
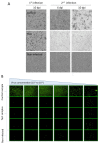
Adenoviruses are promising vectors for vaccine production and gene therapy. Despite all the efforts in removing animal-derived components such as fetal bovine serum (FBS) during the production of adenovirus vector (AdV), FBS is still frequently employed in the early stages of production. Conventionally, first-generation AdVs (E1 deleted) are generated in different variants of adherent HEK293 cells, and plaque purification (if needed) is performed in adherent cell lines in the presence of FBS. In this study, we generated an AdV stock in SF-BMAdR (A549 cells adapted to suspension culture in serum-free medium). We also developed a limiting dilution method using the same cell line to replace the plaque purification assay. By combining these two technologies, we were able to completely remove the need for FBS from the process of generating and producing AdVs. In addition, we demonstrated that the purified AdV stock is free of any replication-competent adenovirus (RCA). Furthermore, we demonstrated that our limiting dilution method could effectively rescue an AdV from a stock that is highly contaminated with RCA.
Keywords: adenovirus vector; animal-origin-free culture media; limiting dilution; replication-competent adenovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Establishment and validation of new complementing cells for production of E1-deleted adenovirus vectors in serum-free suspension culture.J Virol Methods. 2014 Nov;208:177-88. doi: 10.1016/j.jviromet.2014.08.013. Epub 2014 Aug 23. J Virol Methods. 2014. PMID: 25159033
-
Complementary Cell Lines for Protease Gene-Deleted Single-Cycle Adenovirus Vectors.Cells. 2023 Feb 14;12(4):619. doi: 10.3390/cells12040619. Cells. 2023. PMID: 36831286 Free PMC article.
-
Human amniocyte-derived cells are a promising cell host for adenoviral vector production under serum-free conditions.Biotechnol J. 2015 May;10(5):760-71. doi: 10.1002/biot.201400765. Biotechnol J. 2015. PMID: 25943527
-
Adenoviral Gene Therapy Vectors in Clinical Use-Basic Aspects with a Special Reference to Replication-Competent Adenovirus Formation and Its Impact on Clinical Safety.Int J Mol Sci. 2023 Nov 20;24(22):16519. doi: 10.3390/ijms242216519. Int J Mol Sci. 2023. PMID: 38003709 Free PMC article. Review.
-
[Recent progress in adenovirus vectors: focusing on VA-deleted AdV].Uirusu. 2013;63(2):155-64. doi: 10.2222/jsv.63.155. Uirusu. 2013. PMID: 25366050 Review. Japanese.
Cited by
-
Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications.Viruses. 2024 Feb 28;16(3):377. doi: 10.3390/v16030377. Viruses. 2024. PMID: 38543743 Free PMC article. Review.
References
-
- World Health Organization COVID-19 Vaccine Tracker and Landscape. [(accessed on 7 June 2023)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

