Chemoprophylactic Assessment of Combined Intranasal SARS-CoV-2 Polymerase and Exonuclease Inhibition in Syrian Golden Hamsters
- PMID: 38005839
- PMCID: PMC10675045
- DOI: 10.3390/v15112161
Chemoprophylactic Assessment of Combined Intranasal SARS-CoV-2 Polymerase and Exonuclease Inhibition in Syrian Golden Hamsters
Abstract
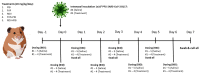
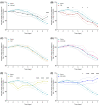
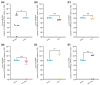
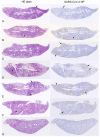
Pibrentasvir (PIB) has been demonstrated to block exonuclease activity of the SARS-CoV-2 polymerase, protecting favipiravir (FVP) and remdesivir (RDV) from post-incorporation excision and eliciting antiviral synergy in vitro. The present study investigated the chemoprophylactic efficacy of PIB, FVP, RDV, FVP with PIB, or RDV with PIB dosed intranasally twice a day, using a Syrian golden hamster contact transmission model. Compared to the saline control, viral RNA levels were significantly lower in throat swabs in FVP (day 7), RDV (day 3, 5, 7), and RDV+PIB (day 3, 5) treatment groups. Similarly, findings were evident for nasal turbinate after PIB and RDV treatment, and lungs after PIB, FVP, and FVP+PIB treatment at day 7. Lung viral RNA levels after RDV and RDV+PIB treatment were only detectable in two animals per group, but the overall difference was not statistically significant. In situ examination of the lungs confirmed SARS-CoV-2 infection in all animals, except for one in each of the RDV and RDV+PIB treatment groups, which tested negative in all virus detection approaches. Overall, prevention of transmission was observed in most animals treated with RDV, while other agents reduced the viral load following contact transmission. No benefit of combining FVP or RDV with PIB was observed.
Keywords: SARS-CoV-2; chemoprophylaxis; favipiravir; pibrentasvir; remdesivir.
Conflict of interest statement
A.O. is a director of Tandem Nano Ltd. and co-inventor of drug delivery patents. A.O. has been co-investigator on funding received by the University of Liverpool from ViiV Healthcare and Gilead Sciences in the past 3 years, unrelated to COVID-19. A.O. has received personal fees from Gilead and Assembly Biosciences in the past 3 years, also unrelated to COVID-19. J.P.S. has received funding from Byotrol Technologies, ENA Respiratory, Sisaf, and Zucero not related to the current abstract.
Figures


Similar articles
-
Assessment of Favipiravir and Remdesivir in Combination for SARS-CoV-2 Infection in Syrian Golden Hamsters.Viruses. 2024 Nov 27;16(12):1838. doi: 10.3390/v16121838. Viruses. 2024. PMID: 39772148 Free PMC article.
-
Co-administration of Favipiravir and the Remdesivir Metabolite GS-441524 Effectively Reduces SARS-CoV-2 Replication in the Lungs of the Syrian Hamster Model.mBio. 2021 Feb 22;13(1):e0304421. doi: 10.1128/mbio.03044-21. Epub 2022 Feb 1. mBio. 2021. PMID: 35100870 Free PMC article.
-
Evaluation of Nafamostat as Chemoprophylaxis for SARS-CoV-2 Infection in Hamsters.Viruses. 2023 Aug 15;15(8):1744. doi: 10.3390/v15081744. Viruses. 2023. PMID: 37632086 Free PMC article.
-
Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir.Biomed Pharmacother. 2022 Mar;147:112700. doi: 10.1016/j.biopha.2022.112700. Epub 2022 Feb 4. Biomed Pharmacother. 2022. PMID: 35131656 Free PMC article. Review.
-
Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis.Virol J. 2020 Sep 24;17(1):141. doi: 10.1186/s12985-020-01412-z. Virol J. 2020. PMID: 32972430 Free PMC article.
Cited by
-
Trypstatin as a Novel TMPRSS2 Inhibitor with Broad-Spectrum Efficacy against Corona and Influenza Viruses.Adv Sci (Weinh). 2025 Jul;12(25):e2506430. doi: 10.1002/advs.202506430. Epub 2025 May 14. Adv Sci (Weinh). 2025. PMID: 40365759 Free PMC article.
-
Assessment of Favipiravir and Remdesivir in Combination for SARS-CoV-2 Infection in Syrian Golden Hamsters.Viruses. 2024 Nov 27;16(12):1838. doi: 10.3390/v16121838. Viruses. 2024. PMID: 39772148 Free PMC article.
References
-
- Dhama K., Nainu F., Frediansyah A., Yatoo M.I., Mohapatra R.K., Chakraborty S., Zhou H., Islam M.R., Mamada S.S., Kusuma H.I., et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health. 2023;16:4–14. doi: 10.1016/j.jiph.2022.11.024. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

