Patterns of Circulating Cytokines and Vascular Markers' Response in the Presence of COVID-19 in Kidney Transplant Recipients Compared with Non-Transplanted Patients
- PMID: 38005844
- PMCID: PMC10675241
- DOI: 10.3390/v15112166
Patterns of Circulating Cytokines and Vascular Markers' Response in the Presence of COVID-19 in Kidney Transplant Recipients Compared with Non-Transplanted Patients
Abstract
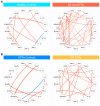
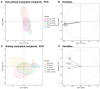
COVID-19's severity has been associated with a possible imbalance in the cross-regulation of cytokines and vascular mediators. Since the beginning of the pandemic, kidney transplant recipients (KTRs) have been identified as patients of high vulnerability to more severe diseases. Thus, aiming to describe the patterns of cytokines and vascular mediators and to trace patients' differences according to their KTR status, this prospective study enrolled 67 COVID-19 patients (20 KTRs) and 29 non-COVID-19 controls before vaccination. A panel comprising 17 circulating cytokines and vascular mediators was run on samples collected at different time points. The cytokine and mediator patterns were investigated via principal component analysis (PCA) and correlation-based network (CBN). In both groups, compared to their respective controls, COVID-19 was associated with higher levels of cytokines and vascular mediators. Differentiating between the KTRs and non-KTRs, the number of correlations was much higher in the non-KTRs (44 vs. 14), and the node analysis showed the highest interactions of NGAL and sVCAM-1 in the non-KTRs and KTRs (9 vs. 4), respectively. In the PCA, while the non-KTRs with COVID-19 were differentiated from their controls in their IL-10, IFN-α, and TNF-α, this pattern was marked in the NGAL, sVCAM-1, and IL-8 of the KTRs.
Keywords: SARS-CoV-2; correlation-based network; cytokines; kidney transplant recipients; vascular mediators.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bertrand D., Hamzaoui M., Lemee V., Lamulle J., Laurent C., Etienne I., Lemoine M., Lebourg L., Hanoy M., Le Roy F., et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021;100:1337–1340. doi: 10.1016/j.kint.2021.09.014. - DOI - PMC - PubMed
-
- Callaghan C.J., Mumford L., Curtis R.M.K., Williams S.V., Whitaker H., Andrews N., Lopez Bernal J., Ushiro-Lumb I., Pettigrew G.J., Thorburn D., et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients. Transplantation. 2022;106:436. doi: 10.1097/TP.0000000000004059. - DOI - PMC - PubMed
-
- Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben-Yehoyada M., Shashar M., Katchman E., Halperin T., Turner D., Goykhman Y., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

