Naringenin Improves Innate Immune Suppression after PRRSV Infection by Reactivating the RIG-I-MAVS Signaling Pathway, Promoting the Production of IFN-I
- PMID: 38005850
- PMCID: PMC10674737
- DOI: 10.3390/v15112172
Naringenin Improves Innate Immune Suppression after PRRSV Infection by Reactivating the RIG-I-MAVS Signaling Pathway, Promoting the Production of IFN-I
Abstract
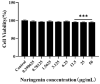
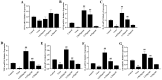
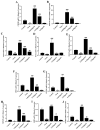
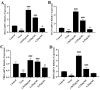
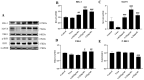
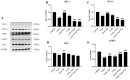
Porcine reproductive and respiratory syndrome (PRRS) has been prevalent for nearly forty years since it was first reported. It has been one of the major diseases jeopardizing the healthy development of the world swine industry, as well as causing great economic losses to the industry's economic development. Furthermore, no way has been found to combat the disease due to the immunosuppressive properties of its pathogen porcine reproductive and respiratory syndrome virus (PRRSV) infection. We previously examined the mRNA expression of IFN-I in PRRSV-infected Marc-145 cells at different time periods using qRT-PCR, and found that the mRNA expression of IFN-I in the late stage of PRRSV infection showed suppression. Naringenin is a flavonoid found in citrus fruits and has a very wide range of pharmacological activities. Therefore, the aim of the present study was to investigate the modulatory effect of naringenin on the suppressed innate immune response after PRRSV infection. The expression of IFN-I, IL-10, and ISGs in the late stage of PRRSV infection was examined using qRT-PCR, and the results showed that naringenin improved the expression of antiviral cytokines suppressed by PRRSV infection. Further results showed that naringenin treatment significantly up-regulated the expression of proteins related to the RIG-I-MAV immune signaling pathway, and that naringenin could not significantly activate the RIG-I-MAVS signaling pathway after the addition of the RIG-I inhibitor Cyclo. Overall, these data demonstrated that naringenin could improve the innate immune response suppressed by PRRSV infection by modulating the RIG-I-MAVS signaling pathway. Therefore, our study will provide a theoretical basis for the development of naringenin as a drug against immunosuppressive viral infectious disease infections.
Keywords: PRRSV; RIG-I-MAVS; innate immune suppression; naringenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
PRRSV non-structural protein 5 inhibits antiviral innate immunity by degrading multiple proteins of RLR signaling pathway through FAM134B-mediated ER-phagy.J Virol. 2024 Oct 22;98(10):e0081624. doi: 10.1128/jvi.00816-24. Epub 2024 Sep 12. J Virol. 2024. PMID: 39264156 Free PMC article.
-
Identification of the RNA Pseudoknot within the 3' End of the Porcine Reproductive and Respiratory Syndrome Virus Genome as a Pathogen-Associated Molecular Pattern To Activate Antiviral Signaling via RIG-I and Toll-Like Receptor 3.J Virol. 2018 May 29;92(12):e00097-18. doi: 10.1128/JVI.00097-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618647 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-κB essential modulator.J Virol. 2014 Sep;88(18):10934-45. doi: 10.1128/JVI.01396-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008936 Free PMC article.
-
Antagonizing interferon-mediated immune response by porcine reproductive and respiratory syndrome virus.Biomed Res Int. 2014;2014:315470. doi: 10.1155/2014/315470. Epub 2014 Jul 3. Biomed Res Int. 2014. PMID: 25101271 Free PMC article. Review.
-
Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus.Viruses. 2012 Apr;4(4):424-46. doi: 10.3390/v4040424. Epub 2012 Apr 2. Viruses. 2012. PMID: 22590680 Free PMC article. Review.
Cited by
-
Anticancer Plant Secondary Metabolites Evicting Linker Histone H1.2 from Chromatin Activate Type I Interferon Signaling.Int J Mol Sci. 2025 Jan 4;26(1):375. doi: 10.3390/ijms26010375. Int J Mol Sci. 2025. PMID: 39796235 Free PMC article.
-
Mechanism of PRRSV infection and antiviral role of polyphenols.Virulence. 2024 Dec;15(1):2417707. doi: 10.1080/21505594.2024.2417707. Epub 2024 Oct 21. Virulence. 2024. PMID: 39432383 Free PMC article. Review.
-
Exploring the Mechanisms for Virus Invasion at the Barrier of Host Defense Involving Signaling Pathways.Viruses. 2024 Dec 19;16(12):1939. doi: 10.3390/v16121939. Viruses. 2024. PMID: 39772245 Free PMC article.
References
-
- Senthilkumar D., Rajukumar K., Kumar M., Kalaiyarasu S., Shrivastava D., Katare M., Kulkarni D., Singh V. Porcine reproductive and respiratory syndrome virus induces concurrent elevation of High Mobility Group Box-1 protein and pro-inflammatory cytokines in experimentally infected piglets. Cytokine. 2019;113:21–30. doi: 10.1016/j.cyto.2018.06.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

