Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses
- PMID: 38005871
- PMCID: PMC10674894
- DOI: 10.3390/v15112194
Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses
Abstract
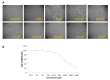
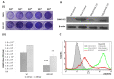
Alphaviruses are serious zoonotic threats responsible for significant morbidity, causing arthritis or encephalitis. So far, no licensed drugs or vaccines are available to combat alphaviral infections. About 300,000 chikungunya virus (CHIKV) infections have been reported in 2023, with more than 300 deaths, including reports of a few cases in the USA as well. The discovery and development of small-molecule drugs have been revolutionized over the last decade. Here, we employed a cell-based screening approach using a series of in-house small-molecule libraries to test for their ability to inhibit CHIKV replication. DCR 137, a quinazoline derivative, was found to be the most potent inhibitor of CHIKV replication in our screening assay. Both, the cytopathic effect, and immunofluorescence of infected cells were reduced in a dose-dependent manner with DCR 137 post-treatment. Most importantly, DCR 137 was more protective than the traditional ribavirin drug and reduced CHIKV plaque-forming units by several log units. CHIKV-E2 protein levels were also reduced in a dose-dependent manner. Further, DCR 137 was probed for its antiviral activity against another alphavirus, the Ross River virus, which revealed effective inhibition of viral replication. These results led to the identification of a potential quinazoline candidate for future optimization that might act as a pan-alphavirus inhibitor.
Keywords: 2,4-diaminoquinazoline derivative; Ross River virus; antiviral drug; chikungunya virus; drug screening; pan-alphavirus inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Antagonism of the Sodium-Potassium ATPase Impairs Chikungunya Virus Infection.mBio. 2016 May 24;7(3):e00693-16. doi: 10.1128/mBio.00693-16. mBio. 2016. PMID: 27222471 Free PMC article.
-
Novel Mutations in nsP2 Abolish Chikungunya Virus-Induced Transcriptional Shutoff and Make the Virus Less Cytopathic without Affecting Its Replication Rates.J Virol. 2019 Feb 5;93(4):e02062-18. doi: 10.1128/JVI.02062-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487275 Free PMC article.
-
Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses.Antiviral Res. 2016 Feb;126:117-24. doi: 10.1016/j.antiviral.2015.12.012. Epub 2016 Jan 2. Antiviral Res. 2016. PMID: 26752081
-
Antivirals against the Chikungunya Virus.Viruses. 2021 Jul 5;13(7):1307. doi: 10.3390/v13071307. Viruses. 2021. PMID: 34372513 Free PMC article. Review.
-
Antiviral drug discovery against arthritogenic alphaviruses: Tools and molecular targets.Biochem Pharmacol. 2020 Apr;174:113777. doi: 10.1016/j.bcp.2019.113777. Epub 2019 Dec 23. Biochem Pharmacol. 2020. PMID: 31874146 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

