Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing
- PMID: 38005876
- PMCID: PMC10675624
- DOI: 10.3390/v15112198
Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing
Abstract
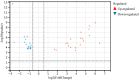
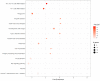
Influenza A virus (IAV) is known to cause mild to severe respiratory illness. Under some conditions, the infection can lead to pneumonia (viral or bacterial), acute respiratory distress syndrome, and other complications that can be fatal, especially in vulnerable populations such as the elderly, young children, and individuals with underlying health conditions. Despite previous studies, little is known about the host immune response and neuroimmune interactions in IAV infection. Using RNA sequencing, we performed transcriptomic analysis of murine lung tissue 21 days post infection (dpi) with IAV (H1N1) in order to find the differentially expression genes (DEGs) related to the host immune response and neuroimmune interactions inside the lung during recovery. Among 792 DEGs, 434 genes were up-regulated, whereas 358 genes were down-regulated. The most prominent molecular functions of the up-regulated genes were related to the immune response and tissue repair, whereas a large proportion of the down-regulated genes were associated with neural functions. Although further molecular/functional studies need to be performed for these DEGs, our results facilitate the understanding of the host response (from innate immunity to adaptive immunity) and neuroimmune interactions in infected lungs at the recovery stage of IAV infection. These genes might have potential uses as mechanistic/diagnostic biomarkers and represent possible targets for anti-IAV therapies.
Keywords: RNA sequencing; immunometabolism; influenza A virus; neuroregulation; respiratory infection; transcriptomic analysis.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
RNA-Seq Analysis of Influenza A Virus-Induced Transcriptional Changes in Mice Lung and Its Possible Implications for the Virus Pathogenicity in Mice.Viruses. 2021 Oct 8;13(10):2031. doi: 10.3390/v13102031. Viruses. 2021. PMID: 34696461 Free PMC article.
-
Intranasal delivery of Duox2 DNA using cationic polymer can prevent acute influenza A viral infection in vivo lung.Appl Microbiol Biotechnol. 2018 Jan;102(1):105-115. doi: 10.1007/s00253-017-8512-1. Epub 2017 Sep 21. Appl Microbiol Biotechnol. 2018. PMID: 28936773
-
Suppression of Cytotoxic T Cell Functions and Decreased Levels of Tissue-Resident Memory T Cells during H5N1 Infection.J Virol. 2020 Apr 16;94(9):e00057-20. doi: 10.1128/JVI.00057-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32075925 Free PMC article.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
RNA N6-methyladenosine methylation in influenza A virus infection.Front Microbiol. 2024 Jun 18;15:1401997. doi: 10.3389/fmicb.2024.1401997. eCollection 2024. Front Microbiol. 2024. PMID: 38957616 Free PMC article. Review.
Cited by
-
SARS-CoV-2 and Other Respiratory Viruses in Human Olfactory Pathophysiology.Microorganisms. 2024 Mar 7;12(3):540. doi: 10.3390/microorganisms12030540. Microorganisms. 2024. PMID: 38543591 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

