Crystal Structure of Inhibitor-Bound GII.4 Sydney 2012 Norovirus 3C-Like Protease
- PMID: 38005879
- PMCID: PMC10674469
- DOI: 10.3390/v15112202
Crystal Structure of Inhibitor-Bound GII.4 Sydney 2012 Norovirus 3C-Like Protease
Abstract
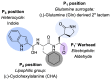
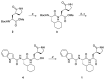
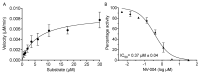
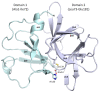
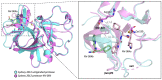
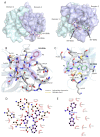
Norovirus is the leading cause of viral gastroenteritis worldwide, and there are no approved vaccines or therapeutic treatments for chronic or severe norovirus infections. The structural characterisation of the norovirus protease and drug development has predominantly focused upon GI.1 noroviruses, despite most global outbreaks being caused by GII.4 noroviruses. Here, we determined the crystal structures of the GII.4 Sydney 2012 ligand-free norovirus protease at 2.79 Å and at 1.83 Å with a covalently bound high-affinity (IC50 = 0.37 µM) protease inhibitor (NV-004). We show that the active sites of the ligand-free protease structure are present in both open and closed conformations, as determined by their Arg112 side chain orientation. A comparative analysis of the ligand-free and ligand-bound protease structures reveals significant structural differences in the active site cleft and substrate-binding pockets when an inhibitor is covalently bound. We also report a second molecule of NV-004 non-covalently bound within the S4 substrate binding pocket via hydrophobic contacts and a water-mediated hydrogen bond. These new insights can guide structure-aided drug design against the GII.4 genogroup of noroviruses.
Keywords: 3C-like protease; X-ray structure; antiviral; ligand-free protease; norovirus; protease inhibitor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Koo H.L., Neill F.H., Estes M.K., Munoz F.M., Cameron A., DuPont H.L., Atmar R.L. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J. Pediatric Infect. Dis. Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

