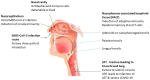
COVID-19 Vaccines for Optimizing Immunity in the Upper Respiratory Tract
- PMID: 38005881
- PMCID: PMC10674974
- DOI: 10.3390/v15112203
COVID-19 Vaccines for Optimizing Immunity in the Upper Respiratory Tract
Abstract
Rapid development and deployment of vaccines greatly reduced mortality and morbidity during the COVID-19 pandemic. The most widely used COVID-19 vaccines approved by national regulatory authorities require intramuscular administration. SARS-CoV-2 initially infects the upper respiratory tract, where the infection can be eliminated with little or no symptoms by an effective immune response. Failure to eliminate SARS-CoV-2 in the upper respiratory tract results in lower respiratory tract infections that can lead to severe disease and death. Presently used intramuscularly administered COVID-19 vaccines are effective in reducing severe disease and mortality, but are not entirely able to prevent asymptomatic and mild infections as well as person-to-person transmission of the virus. Individual and population differences also influence susceptibility to infection and the propensity to develop severe disease. This article provides a perspective on the nature and the mode of delivery of COVID-19 vaccines that can optimize protective immunity in the upper respiratory tract to reduce infections and virus transmission as well as severe disease.
Keywords: COVID-19; COVID-19 vaccines; SARS-CoV-2; adaptive immunity to COVID-19; clinical vaccine trials; innate immunity to COVID-19; mucosal vaccines; nasal vaccines; upper respiratory tract immunity; vaccine safety.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines.Trends Mol Med. 2023 Apr;29(4):255-267. doi: 10.1016/j.molmed.2023.01.003. Epub 2023 Jan 23. Trends Mol Med. 2023. PMID: 36764906 Free PMC article. Review.
-
Innate and Adaptive Immune Responses in the Upper Respiratory Tract and the Infectivity of SARS-CoV-2.Viruses. 2022 Apr 29;14(5):933. doi: 10.3390/v14050933. Viruses. 2022. PMID: 35632675 Free PMC article. Review.
-
Intranasal COVID-19 vaccines: From bench to bed.EBioMedicine. 2022 Feb;76:103841. doi: 10.1016/j.ebiom.2022.103841. Epub 2022 Jan 24. EBioMedicine. 2022. PMID: 35085851 Free PMC article. Review.
-
Intranasal administration of a single dose of MVA-based vaccine candidates against COVID-19 induced local and systemic immune responses and protects mice from a lethal SARS-CoV-2 infection.Front Immunol. 2022 Sep 12;13:995235. doi: 10.3389/fimmu.2022.995235. eCollection 2022. Front Immunol. 2022. PMID: 36172368 Free PMC article.
-
Intranasal inoculation of an MVA-based vaccine induces IgA and protects the respiratory tract of hACE2 mice from SARS-CoV-2 infection.Proc Natl Acad Sci U S A. 2022 Jun 14;119(24):e2202069119. doi: 10.1073/pnas.2202069119. Epub 2022 Jun 9. Proc Natl Acad Sci U S A. 2022. PMID: 35679343 Free PMC article.
Cited by
-
Immune Stimulation with Imiquimod to Best Face SARS-CoV-2 Infection and Prevent Long COVID.Int J Mol Sci. 2024 Jul 12;25(14):7661. doi: 10.3390/ijms25147661. Int J Mol Sci. 2024. PMID: 39062904 Free PMC article. Review.
-
The effect of COVID-19 vaccination on symptomatic infection and related symptoms among preterm-born children aged 3-7 years in China.Sci Rep. 2024 Oct 25;14(1):25384. doi: 10.1038/s41598-024-76609-1. Sci Rep. 2024. PMID: 39455727 Free PMC article.
References
-
- World Health Organization Coronavirus (COVID-19) Dashboard. [(accessed on 4 October 2023)];2023 Available online: https://covid19.who.int/
-
- Yamana T.K., Galanti M., Pei S., Di Fusco M., Angulo F.J., Moran M.M., Khan F., Swerdlow D.L., Shaman J. The impact of COVID-19 vaccination in the US: Averted burden of SARS-CoV-2-related cases, hospitalizations and deaths. PLoS ONE. 2023;18:e0275699. doi: 10.1371/journal.pone.0275699. - DOI - PMC - PubMed
-
- Haas E.J., McLaughlin J.M., Khan F., Angulo F.J., Anis E., Lipsitch M., Singer S.R., Mircus G., Brooks N., Smaja M., et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: A retrospective surveillance study. Lancet Infect. Dis. 2022;22:357–366. doi: 10.1016/S1473-3099(21)00566-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous