Seneca Valley Virus Degrades STING via PERK and ATF6-Mediated Reticulophagy
- PMID: 38005886
- PMCID: PMC10674438
- DOI: 10.3390/v15112209
Seneca Valley Virus Degrades STING via PERK and ATF6-Mediated Reticulophagy
Abstract
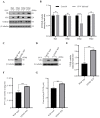
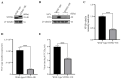
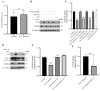
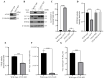
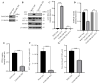
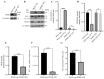
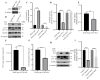
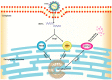
Seneca Valley Virus (SVV), a member of the Picornaviridae family, is an emerging porcine virus that can cause vesicular disease in pigs. However, the immune evasion mechanism of SVV remains unclear, as does its interaction with other pathways. STING (Stimulator of interferon genes) is typically recognized as a critical factor in innate immune responses to DNA virus infection, but its role during SVV infection remains poorly understood. In the present study, we observed that STING was degraded in SVV-infected PK-15 cells, and SVV replication in the cells was affected when STING was knockdown or overexpressed. The STING degradation observed was blocked when the SVV-induced autophagy was inhibited by using autophagy inhibitors (Chloroquine, Bafilomycin A1) or knockdown of autophagy related gene 5 (ATG5), suggesting that SVV-induced autophagy is responsible for STING degradation. Furthermore, the STING degradation was inhibited when reticulophagy regulator 1 (FAM134B), a reticulophagy related receptor, was knocked down, indicating that SVV infection induces STING degradation via reticulophagy. Further study showed that in eukaryotic translation initiation factor 2 alpha kinase 3 (PERK)/activating transcription factor 6 (ATF6) deficient cells, SVV infection failed to induce reticulophagy-medaited STING degradation, indicating that SVV infection caused STING degradation via PERK/ATF6-mediated reticulophagy. Notably, blocking reticulophagy effectively hindered SVV replication. Overall, our study suggested that SVV infection resulted in STING degradation via PERK and ATF6-mediated reticulophagy, which may be an immune escape strategy of SVV. This finding improves the understanding of the intricate interplay between viruses and their hosts and provides a novel strategy for the development of novel antiviral drugs.
Keywords: STING; SVV; UPR; autophagy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Reticulophagy and viral infection.Autophagy. 2025 Jan;21(1):3-20. doi: 10.1080/15548627.2024.2414424. Epub 2024 Oct 23. Autophagy. 2025. PMID: 39394962 Free PMC article. Review.
-
Seneca valley virus activates autophagy through the PERK and ATF6 UPR pathways.Virology. 2019 Nov;537:254-263. doi: 10.1016/j.virol.2019.08.029. Epub 2019 Aug 30. Virology. 2019. PMID: 31539773
-
Synergetic Contributions of Viral VP1, VP3, and 3C to Activation of the AKT-AMPK-MAPK-MTOR Signaling Pathway for Seneca Valley Virus-Induced Autophagy.J Virol. 2022 Jan 26;96(2):e0155021. doi: 10.1128/JVI.01550-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757844 Free PMC article.
-
Seneca Valley virus induces mitochondrial apoptosis by activating ER stress or the PERK pathway based on Ca2+ transfer from ER to mitochondria.J Virol. 2025 Mar 18;99(3):e0217724. doi: 10.1128/jvi.02177-24. Epub 2025 Feb 6. J Virol. 2025. PMID: 39912666 Free PMC article.
-
Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles.Autophagy. 2021 Feb;17(2):385-401. doi: 10.1080/15548627.2020.1725377. Epub 2020 Feb 12. Autophagy. 2021. PMID: 32048886 Free PMC article. Review.
Cited by
-
Endoplasmic reticulum stress in diseases.MedComm (2020). 2024 Aug 26;5(9):e701. doi: 10.1002/mco2.701. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39188936 Free PMC article. Review.
-
Reticulophagy and viral infection.Autophagy. 2025 Jan;21(1):3-20. doi: 10.1080/15548627.2024.2414424. Epub 2024 Oct 23. Autophagy. 2025. PMID: 39394962 Free PMC article. Review.
-
The cGAS-STING/PERK-eIF2α: Individual or Potentially Collaborative Signaling Transduction in Cardiovascular Diseases.Int J Biol Sci. 2024 Oct 28;20(15):5868-5887. doi: 10.7150/ijbs.101247. eCollection 2024. Int J Biol Sci. 2024. PMID: 39664570 Free PMC article. Review.
References
-
- Zhang L., Mo J., Swanson K.V., Wen H., Petrucelli A., Gregory S.M., Zhang Z., Schneider M., Jiang Y., Fitzgerald K.A., et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40:329–341. doi: 10.1016/j.immuni.2014.01.010. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

