Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia
- PMID: 38005896
- PMCID: PMC10675195
- DOI: 10.3390/v15112219
Chemical Peeling Therapy Using Phenol for the Cervico-Vaginal Intraepithelial Neoplasia
Abstract
Objective: This study aimed to validate the use of liquid phenol-based chemical peeling therapy for cervical and vaginal intraepithelial neoplasia (CIN and VaIN, respectively), with the goal of circumventing obstetric complications associated with surgical treatment and to determine the factors associated with treatment resistance. Methods: A total of 483 eligible women diagnosed with CIN, VaIN, or both, participated in this study. Participants underwent phenol-based chemical peeling therapy every 4 weeks until disease clearance. Disease clearance was determined by negative Pap tests for four consecutive weeks or by colposcopy. HPV genotyping was conducted at the onset of the study and after disease clearance in select cases. Our preliminary analysis compared the recurrence and persistence rates between 294 individuals who received phenol-based chemical peeling therapy and 189 untreated patients. Results: At 2 years following diagnosis, persistent disease was observed in 18%, 60%, and 88% of untreated patients with CIN1-3, respectively, and <2% of patients with CIN who received phenol-based chemical peeling therapy. Among 483 participants, 10 immune-suppressed patients required multiple treatments to achieve disease clearance, and 7 were diagnosed with cervical cancer. Of the 466 participants, except those with cancer or immune suppression, the number of treatment sessions until CIN/VaIN clearance ranged from 2 to 42 (average: 9.2 sessions). In total, 43 participants (9.2%) underwent surgical treatment. Six patients (1.3%) experienced recurrence of CIN2 or worse, suggesting that treatment failed in 46 patients (9.9%). No obstetrical complications were noted among the 98 pregnancies following this therapy. Factors associated with resistance to this therapy include immune suppression, ages 35-39 years, higher-grade lesions, and multiple HPV-type infections. Conclusions: Phenol-based therapy is safe and effective for CINs and VaINs. Women aged < 35 years and with persistent CIN1 or CIN2 with a single HPV-type infection are suitable candidates for phenol-based chemical peeling therapy. However, this therapy requires multiple lengthy sessions.
Keywords: CIN; chemical-peeling therapy; human papillomavirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

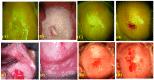
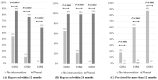

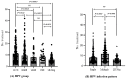
Similar articles
-
Clinical presentation, treatment, and outcomes associated with vaginal intraepithelial neoplasia: A retrospective study of 118 patients.J Obstet Gynaecol Res. 2021 May;47(5):1624-1630. doi: 10.1111/jog.14733. Epub 2021 Mar 23. J Obstet Gynaecol Res. 2021. PMID: 33754436 Review.
-
The prevalence of VAIN, CIN, and related HPV genotypes in Japanese women with abnormal cytology.J Med Virol. 2020 Mar;92(3):364-371. doi: 10.1002/jmv.25611. Epub 2019 Nov 18. J Med Virol. 2020. PMID: 31642536
-
Prediction of recurrence after treatment for high-grade cervical intraepithelial neoplasia: the role of human papillomavirus testing and age at conisation.BJOG. 2006 Nov;113(11):1303-7. doi: 10.1111/j.1471-0528.2006.01063.x. Epub 2006 Sep 15. BJOG. 2006. PMID: 16978225
-
The relationship of human papillomavirus infection with endocervical glandular involvement on cone specimens in women with cervical intraepithelial neoplasia.Gynecol Oncol. 2020 Dec;159(3):630-635. doi: 10.1016/j.ygyno.2020.09.034. Epub 2020 Oct 9. Gynecol Oncol. 2020. PMID: 33041069
-
Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review.Gynecol Oncol. 2015 Nov;139(2):377-84. doi: 10.1016/j.ygyno.2015.08.018. Epub 2015 Aug 31. Gynecol Oncol. 2015. PMID: 26335596 Review.
References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; Lyon, France: 2020. [(accessed on 26 September 2020)]. Available online: https://gco.iarc.fr/today.
-
- Cancer Statistics Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare) for Incidence and Mortality. 2019. [(accessed on 26 September 2023)]. Available online: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statisti....
-
- World Health Organization (WHO) Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization (WHO); Geneva, Switzerland: 2000.
-
- Kyrgiou M., Athanasiou A., Kalliala I.E.J., Paraskevaidi M., Mitra A., Martin-Hirsch P.P.L., Arbyn M., Bennett P., Paraskevaidis E. Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017;2017:CD012847. doi: 10.1002/14651858.CD012847. - DOI - PMC - PubMed

