Genomic Markers Associated with Cytomegalovirus DNAemia in Kidney Transplant Recipients
- PMID: 38005904
- PMCID: PMC10674338
- DOI: 10.3390/v15112227
Genomic Markers Associated with Cytomegalovirus DNAemia in Kidney Transplant Recipients
Abstract
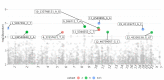
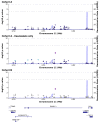
Human cytomegalovirus (CMV) is a major pathogen after solid organ transplantation, leading to high morbidity and mortality. Transplantation from a CMV-seropositive donor to a CMV-seronegative recipient (D+/R-) is associated with high risk of CMV disease. However, that risk is not uniform, suggesting a role for host factors in immune control of CMV. To identify host genetic factors that control CMV DNAemia post transplantation, we performed a whole-exome association study in two cohorts of D+/R- kidney transplant recipients. Quantitative CMV DNA was measured for at least one year following transplantation. Several CMV-protective single-nucleotide polymorphisms (SNPs) were identified in the first cohort (72 patients) but were not reproducible in the second cohort (126 patients). A meta-analysis of both cohorts revealed several SNPs that were significantly associated with protection from CMV DNAemia. The copy number variation of several genes was significantly different between recipients with and without CMV DNAemia. Amongst patients with CMV DNAemia in the second cohort, several variants of interest (p < 5 × 10-5), the most common of which was NLRC5, were associated with peak viral load. We provide new predictive genetic markers for protection of CMV DNAemia. These markers should be validated in larger cohorts.
Keywords: CMV DNAemia; genetic susceptibility; human cytomegalovirus; kidney transplantation; whole-exome sequencing.
Conflict of interest statement
The authors report no conflicts of interest. Robin Avery has been an investigator on studies funded by Aicuris (Wuppertal, Germany), Astellas (Tokyo, Japan), Astra-Zeneca (Tokyo, Japan), Chimerix (Durham, NC, USA), Merck (Darmstadt, Germany), Oxford Immunotec (Oxfordshire, UK), Qiagen (Hong Kong, China), Regeneron (New York, NY, USA), and Takeda (Tokyo, Japan). No personal financial remuneration was received; study/grant support only.
Figures
Similar articles
-
Incidence of Cytomegalovirus DNAemia in Pediatric Kidney Transplant Recipients After Cessation of Antiviral Prophylaxis.Transplantation. 2018 Aug;102(8):1391-1396. doi: 10.1097/TP.0000000000002115. Transplantation. 2018. PMID: 29377877
-
Predictive factors of spontaneous CMV DNAemia clearance in kidney transplantation.J Clin Virol. 2018 Feb-Mar;99-100:38-43. doi: 10.1016/j.jcv.2017.12.011. Epub 2017 Dec 29. J Clin Virol. 2018. PMID: 29306112
-
Impact of an ultrasensitive Cytomegalovirus quantitative nucleic acid test on Cytomegalovirus detection and therapy in renal transplant recipients.Transpl Infect Dis. 2024 Feb;26(1):e14219. doi: 10.1111/tid.14219. Epub 2023 Dec 30. Transpl Infect Dis. 2024. PMID: 38158932 Free PMC article.
-
GCV/VCVG prophylaxis against CMV DNAemia in pediatric renal transplant patients: A systematic review and meta-analysis.Pediatr Transplant. 2019 Sep;23(6):e13514. doi: 10.1111/petr.13514. Epub 2019 Jun 18. Pediatr Transplant. 2019. PMID: 31210393
-
Update on cytomegalovirus in transplant recipients: new agents, prophylaxis, and cell-mediated immunity.Curr Opin Infect Dis. 2021 Aug 1;34(4):307-313. doi: 10.1097/QCO.0000000000000746. Curr Opin Infect Dis. 2021. PMID: 34074879 Review.
Cited by
-
Real-world safety of maribavir: a retrospective study based on signal detection in the FDA adverse event reporting system.Int J Clin Pharm. 2025 Jun;47(3):767-774. doi: 10.1007/s11096-025-01869-4. Epub 2025 Jan 17. Int J Clin Pharm. 2025. PMID: 39821007
References
-
- Kovacs A., Schluchter M., Easley K., Demmler G., Shearer W., La R.P., Pitt J., Cooper E., Goldfarb J., Hodes D., et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N. Engl. J. Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. - DOI - PMC - PubMed
-
- Kotton C.N., Kumar D., Caliendo A.M., Huprikar S., Chou S., Danziger-Isakov L., Humar A., The Transplantation Society International CMV Consensus Group The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900–931. doi: 10.1097/TP.0000000000002191. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

