The Application of Prodrugs as a Tool to Enhance the Properties of Nucleoside Reverse Transcriptase Inhibitors
- PMID: 38005911
- PMCID: PMC10675571
- DOI: 10.3390/v15112234
The Application of Prodrugs as a Tool to Enhance the Properties of Nucleoside Reverse Transcriptase Inhibitors
Abstract
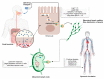
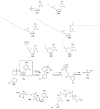
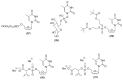
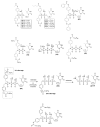
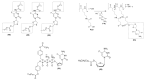
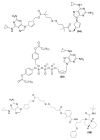
Antiretroviral Therapy (ART) is an effective treatment for human immunodeficiency virus (HIV) which has transformed the highly lethal disease, acquired immunodeficiency syndrome (AIDS), into a chronic and manageable condition. However, better methods need to be developed for enhancing patient access and adherence to therapy and for improving treatment in the long term to reduce adverse effects. From the perspective of drug discovery, one promising strategy is the development of anti-HIV prodrugs. This approach aims to enhance the efficacy and safety of treatment, promoting the development of more appropriate and convenient systems for patients. In this review, we discussed the use of the prodrug approach for HIV antiviral agents and emphasized nucleoside reverse transcriptase inhibitors. We comprehensively described various strategies that are used to enhance factors such as water solubility, bioavailability, pharmacokinetic parameters, permeability across biological membranes, chemical stability, drug delivery to specific sites/organs, and tolerability. These strategies might help researchers conduct better studies in this field. We also reported successful examples from the primary therapeutic classes while discussing the advantages and limitations. In this review, we highlighted the key trends in the application of the prodrug approach for treating HIV/AIDS.
Keywords: AIDS; HIV; codrugs; medicinal chemistry; new drugs; nucleoside reverse transcriptase inhibitors (NRTIs); prodrugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

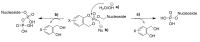
Similar articles
-
Strategies in the Design and Development of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs).Viruses. 2023 Sep 25;15(10):1992. doi: 10.3390/v15101992. Viruses. 2023. PMID: 37896769 Free PMC article. Review.
-
Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase.Curr Top Med Chem. 2004;4(9):895-919. doi: 10.2174/1568026043388484. Curr Top Med Chem. 2004. PMID: 15134548 Review.
-
Current insights and molecular docking studies of HIV-1 reverse transcriptase inhibitors.Chem Biol Drug Des. 2024 Jan;103(1):e14372. doi: 10.1111/cbdd.14372. Epub 2023 Oct 10. Chem Biol Drug Des. 2024. PMID: 37817296 Review.
-
HIV nucleoside reverse transcriptase inhibitors.Eur J Med Chem. 2022 Oct 5;240:114554. doi: 10.1016/j.ejmech.2022.114554. Epub 2022 Jun 20. Eur J Med Chem. 2022. PMID: 35792384 Review.
-
The nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors in the treatment of HIV infections (AIDS).Adv Pharmacol. 2013;67:317-58. doi: 10.1016/B978-0-12-405880-4.00009-3. Adv Pharmacol. 2013. PMID: 23886005 Review.
Cited by
-
Structure-Activity Relationships of Natural and Semisynthetic Plecomacrolides Suggest Distinct Pathways for HIV-1 Immune Evasion and Vacuolar ATPase-Dependent Lysosomal Acidification.J Med Chem. 2024 Mar 28;67(6):4483-4495. doi: 10.1021/acs.jmedchem.3c01574. Epub 2024 Mar 7. J Med Chem. 2024. PMID: 38452116 Free PMC article.
-
Study of Hydrolysis Kinetics and Synthesis of Single Isomer of Phosphoramidate ProTide-Acyclovir.ACS Omega. 2024 Nov 1;9(45):45221-45231. doi: 10.1021/acsomega.4c06645. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554450 Free PMC article.
-
Current Trends in Clinical Trials of Prodrugs.Pharmaceuticals (Basel). 2025 Feb 4;18(2):210. doi: 10.3390/ph18020210. Pharmaceuticals (Basel). 2025. PMID: 40006024 Free PMC article. Review.
References
-
- Chun H.M., Dirlikov E., Cox M.H., Sherlock M.W., Obeng-Aduasare Y., Sato K., Voetsch A.C., Ater A.D., Romano E.R., Tomlinson H., et al. Vital Signs: Progress Toward Eliminating HIV as a Global Public Health Threat Through Scale-Up of Antiretroviral Therapy and Health System Strengthening Supported by the U.S. President's Emergency Plan for AIDS Relief—Worldwide, 2004–2022. MMWR Morb. Mortal. Wkly. Rep. 2023;72:317–324. doi: 10.15585/mmwr.mm7212e1. - DOI - PMC - PubMed
-
- World Health Organization HIV Data and Statistics. [(accessed on 12 August 2023)]. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/s....
-
- O’Connor J.L., Gardner E.M., Mannheimer S.B., Lifson A.R., Esser S., Telzak E.E., Phillips A.N., INSIGHT SMART Study Group Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J. Infect. Dis. 2013;208:40–49. doi: 10.1093/infdis/jis731. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

