Pathogenicity Studies of NADC34-like Porcine Reproductive and Respiratory Syndrome Virus LNSY-GY and NADC30-like Porcine Reproductive and Respiratory Syndrome Virus GXGG-8011 in Piglets
- PMID: 38005924
- PMCID: PMC10674415
- DOI: 10.3390/v15112247
Pathogenicity Studies of NADC34-like Porcine Reproductive and Respiratory Syndrome Virus LNSY-GY and NADC30-like Porcine Reproductive and Respiratory Syndrome Virus GXGG-8011 in Piglets
Abstract
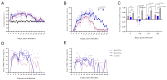
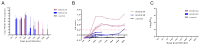
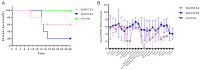
The porcine reproductive and respiratory syndrome virus (PRRSV) has caused significant economic losses to the swine industry. The U.S., China, and Peru have reported NADC30-like or NADC34-like PRRSV-infected piglets, which have been identified as the cause of a significant number of abortions in clinics. Although the pathogenicity of NADC30-like PRRSV and NADC34-like PRRSV in piglets exhibits significant variability globally, studies on their pathogenicity in China are limited. In this study, the animal experiments showed that within 8-14 days post-infection, both piglets infected with NADC30-like PRRSV GXGG-8011 and those infected with NADC34-like PRRSV LNSY-GY exhibited significant weight loss compared to the control piglets. Additionally, the viremia of the LNSY-GY persisted for 28 days, while the viremia of piglets infected with the GXGG-8011 lasted for 17 days. Similarly, the duration of viral shedding through the fecal-oral route after the LNSY-GY infection was longer than that observed after the GXGG-8011 infection. Furthermore, post-infection, both the LNSY-GY and GXGG-8011 led to pronounced histopathological lesions in the lungs of piglets, including interstitial pneumonia and notable viral colonization. However, the antibody production in the LNSY-GY-infected group occurred earlier than that in the GXGG-8011-infected group. Our research findings indicate that LNSY-GY is a mildly pathogenic strain in piglets, whereas we speculate that the GXGG-8011 might be a highly pathogenic strain.
Keywords: NADC30-like; NADC34-like; PRRSV; pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 belongs to sublineage 1.8 (NADC30-like), while LNSY-GY labeled with
belongs to sublineage 1.8 (NADC30-like), while LNSY-GY labeled with  belongs to sublineage 1.5 (NADC34-like).
belongs to sublineage 1.5 (NADC34-like).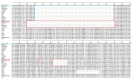

Similar articles
-
Molecular Characteristics and Pathogenicity of a Novel Recombinant Porcine Reproductive and Respiratory Syndrome Virus Strain from NADC30-, NADC34-, and JXA1-Like Strains That Emerged in China.Microbiol Spectr. 2022 Dec 21;10(6):e0266722. doi: 10.1128/spectrum.02667-22. Epub 2022 Nov 10. Microbiol Spectr. 2022. PMID: 36354339 Free PMC article.
-
Comparative Genomic and Biological Investigation of NADC30- and NADC34-Like PRRSV Strains Isolated in South Korea.Transbound Emerg Dis. 2025 Jan 22;2025:9015349. doi: 10.1155/tbed/9015349. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40302751 Free PMC article.
-
Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China.Vet Microbiol. 2020 Jul;246:108727. doi: 10.1016/j.vetmic.2020.108727. Epub 2020 May 16. Vet Microbiol. 2020. PMID: 32605755
-
A recombinant type 2 porcine reproductive and respiratory syndrome virus between NADC30-like and a MLV-like: Genetic characterization and pathogenicity for piglets.Infect Genet Evol. 2017 Oct;54:279-286. doi: 10.1016/j.meegid.2017.07.016. Epub 2017 Jul 13. Infect Genet Evol. 2017. PMID: 28713014
-
High Pathogenicity of a Chinese NADC34-like PRRSV on Pigs.Microbiol Spectr. 2022 Aug 31;10(4):e0154122. doi: 10.1128/spectrum.01541-22. Epub 2022 Jun 29. Microbiol Spectr. 2022. PMID: 35766496 Free PMC article.
Cited by
-
The role of immune checkpoint molecules in PRRSV-2-induced immune modulation: insights from comparative in vivo evaluation including NADC34-like PRRSV.J Virol. 2025 Jul 22;99(7):e0229824. doi: 10.1128/jvi.02298-24. Epub 2025 Jun 3. J Virol. 2025. PMID: 40459260 Free PMC article.
-
Genetic evolution analysis of PRRSV ORF5 gene in five provinces of Northern China in 2024.BMC Vet Res. 2025 Apr 3;21(1):242. doi: 10.1186/s12917-025-04679-y. BMC Vet Res. 2025. PMID: 40176022 Free PMC article.
-
Evaluation of the cross-protective effect of VR2332 modified live virus vaccine against a recombinant NADC34-like porcine reproductive and respiratory syndrome virus.Front Vet Sci. 2024 Nov 20;11:1472960. doi: 10.3389/fvets.2024.1472960. eCollection 2024. Front Vet Sci. 2024. PMID: 39641098 Free PMC article.
-
Epidemiology and genetic characterization of porcine reproductive and respiratory syndrome virus in Fujian Province, China, from 2023 to 2024.Front Vet Sci. 2025 Jul 2;12:1634353. doi: 10.3389/fvets.2025.1634353. eCollection 2025. Front Vet Sci. 2025. PMID: 40671826 Free PMC article.
-
Etiological Detection, Isolation, and Pathogenicity of Porcine Reproductive and Respiratory Syndrome Virus in China.Vet Sci. 2025 May 29;12(6):530. doi: 10.3390/vetsci12060530. Vet Sci. 2025. PMID: 40559767 Free PMC article.
References
-
- Neumann E.J., Kliebenstein J.B., Johnson C.D., Mabry J.W., Bush E.J., Seitzinger A.H., Green A.L., Zimmerman J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

