Effects of Deformed Wing Virus-Targeting dsRNA on Viral Loads in Bees Parasitised and Non-Parasitised by Varroa destructor
- PMID: 38005935
- PMCID: PMC10674661
- DOI: 10.3390/v15112259
Effects of Deformed Wing Virus-Targeting dsRNA on Viral Loads in Bees Parasitised and Non-Parasitised by Varroa destructor
Abstract
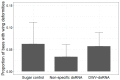
The Varroa destructor mite is a devastating parasite of honey bees; however the negative effects of varroa parasitism are exacerbated by its role as an efficient vector of the honey bee pathogen, Deformed wing virus (DWV). While no direct treatment for DWV infection is available for beekeepers to use on their hives, RNA interference (RNAi) has been widely explored as a possible biopesticide approach for a range of pests and pathogens. This study tested the effectiveness of three DWV-specific dsRNA sequences to lower DWV loads and symptoms in honey bees reared from larvae in laboratory mini-hives containing bees and varroa. The effects of DWV-dsRNA treatment on bees parasitised and non-parasitised by varroa mites during development were investigated. Additionally, the impact of DWV-dsRNA on viral loads and gene expression in brood-parasitising mites was assessed using RNA-sequencing. Bees parasitised during development had significantly higher DWV levels compared to non-parasitised bees. However, DWV-dsRNA did not significantly reduce DWV loads or symptoms in mini-hive reared bees, possibly due to sequence divergence between the DWV variants present in bees and varroa and the specific DWV-dsRNA sequences used. Varroa mites from DWV-dsRNA treated mini-hives did not show evidence of an elevated RNAi response or significant difference in DWV levels. Overall, our findings show that RNAi is not always successful, and multiple factors including pathogen diversity and transmission route may impact its efficiency.
Keywords: RNA interference; RNA-seq; biopesticide; deformed wing virus; double-stranded RNA; varroa destructor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Transmission of deformed wing virus between Varroa destructor foundresses, mite offspring and infested honey bees.Parasit Vectors. 2022 Sep 23;15(1):333. doi: 10.1186/s13071-022-05463-9. Parasit Vectors. 2022. PMID: 36151583 Free PMC article.
-
Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera.J Exp Biol. 2012 Jan 15;215(Pt 2):264-71. doi: 10.1242/jeb.062562. J Exp Biol. 2012. PMID: 22189770
-
Varroa destructor (Mesostigmata: Varroidae) Parasitism and Climate Differentially Influence the Prevalence, Levels, and Overt Infections of Deformed Wing Virus in Honey Bees (Hymenoptera: Apidae).J Insect Sci. 2016 Jun 1;16(1):44. doi: 10.1093/jisesa/iew029. Print 2016. J Insect Sci. 2016. PMID: 27252482 Free PMC article.
-
Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner.Sci Rep. 2019 Aug 27;9(1):12445. doi: 10.1038/s41598-019-47447-3. Sci Rep. 2019. PMID: 31455863 Free PMC article.
-
Emerging and re-emerging viruses of the honey bee (Apis mellifera L.).Vet Res. 2010 Nov-Dec;41(6):54. doi: 10.1051/vetres/2010027. Epub 2010 Apr 29. Vet Res. 2010. PMID: 20423694 Free PMC article. Review.
Cited by
-
The effects of queen mandibular pheromone on nurse-aged honey bee (Apis mellifera) hypopharyngeal gland size and lipid metabolism.PLoS One. 2024 Sep 6;19(9):e0292500. doi: 10.1371/journal.pone.0292500. eCollection 2024. PLoS One. 2024. PMID: 39240896 Free PMC article.
-
The Role of Pathogens in Bumblebee Decline: A Review.Pathogens. 2025 Jan 18;14(1):94. doi: 10.3390/pathogens14010094. Pathogens. 2025. PMID: 39861055 Free PMC article. Review.
-
Deformed wing virus coopts the host arginine kinase to enhance its fitness in honey bees (Apis mellifera).BMC Biol. 2025 Jan 13;23(1):12. doi: 10.1186/s12915-025-02117-x. BMC Biol. 2025. PMID: 39800727 Free PMC article.
References
-
- Chen Y., Pettis J.S., Evans J.D., Kramer M., Feldlaufer M.F. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie. 2004;35:441–448. doi: 10.1051/apido:2004031. - DOI
-
- Ramsey S.D., Ochoa R., Bauchan G., Gulbronson C., Mowery J.D., Cohen A., Lim D., Joklik J., Cicero J.M., Ellis J.D., et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA. 2019;116:1792–1801. doi: 10.1073/pnas.1818371116. - DOI - PMC - PubMed
-
- Santillán-Galicia M.T., Ball B.V., Clark S.J., Alderson P.G. Slow bee paralysis virus and its transmission in honey bee pupae by Varroa destructor. J. Apic. Res. 2014;53:146–154. doi: 10.3896/IBRA.1.53.1.16. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

