Presence and Persistence of Andes Virus RNA in Human Semen
- PMID: 38005942
- PMCID: PMC10675069
- DOI: 10.3390/v15112266
Presence and Persistence of Andes Virus RNA in Human Semen
Abstract
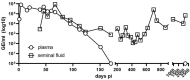
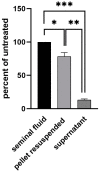
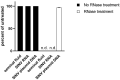
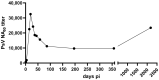
When infecting humans, Andes orthohantavirus (ANDV) may cause a severe disease called hantavirus cardiopulmonary syndrome (HCPS). Following non-specific symptoms, the infection may progress to a syndrome of hemorrhagic fever combined with hyper-acute cardiopulmonary failure. The case fatality rate ranges between 25-40%, depending on the outbreak. In this study, we present the follow-up of a male patient who recovered from HCPS six years ago. We demonstrate that the ANDV genome persists within the reproductive tract for at least 71 months. Genome sequence analysis early and late after infection reveals a low number of mutations (two single nucleotide variants and one deletion), suggesting limited replication activity. We can exclude the integration of the viral genome into the host genome, since the treatment of the specimen with RNAse led to a loss of signal. We demonstrate a long-lasting, strong neutralizing antibody response using pseudovirions expressing the ANDV glycoprotein. Taken together, our results show that ANDV has the potential for sexual transmission.
Keywords: Andes virus; neutralizing antibodies; persistence; semen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

