Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection
- PMID: 38005952
- PMCID: PMC10675108
- DOI: 10.3390/v15112276
Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection
Abstract
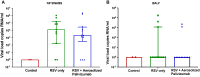
(1) Background: Palivizumab has been an approved preventative monoclonal antibody for respiratory syncytial virus (RSV) infection for over two decades. However, due to its high cost and requirement for multiple intramuscular injections, its use has been limited mostly to high-income countries. Following our previous study showing the successful lung deposition of aerosolised palivizumab in lambs, this current study evaluated the "proof-of-principle" effect of aerosolised palivizumab delivered as a therapeutic to neonatal lambs following RSV infection. (2) Methods: Neonatal lambs were intranasally inoculated with RSV-A2 on day 0 (day 3 post-birth) and treated with aerosolised palivizumab 3 days later (day 3 post-inoculation). Clinical symptoms, RSV viral load and inflammatory response were measured post-inoculation. (3) Results: Aerosolised therapeutic delivery of palivizumab did not reduce RSV viral loads in the nasopharynx nor the bronchoalveolar lavage fluid, but resulted in a modest reduction in inflammatory response at day 6 post-inoculation compared with untreated lambs. (4) Conclusions: This proof-of-principle study shows some evidence of aerosolised palivizumab reducing RSV inflammation, but further studies using optimized protocols are needed in order to validate these findings.
Keywords: aerosolisation; lamb model; lung; palivizumab; respiratory syncytial virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency.Antimicrob Agents Chemother. 2005 Nov;49(11):4700-7. doi: 10.1128/AAC.49.11.4700-4707.2005. Antimicrob Agents Chemother. 2005. PMID: 16251314 Free PMC article.
-
Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model.Antimicrob Agents Chemother. 2004 May;48(5):1811-22. doi: 10.1128/AAC.48.5.1811-1822.2004. Antimicrob Agents Chemother. 2004. PMID: 15105140 Free PMC article.
-
A humanized monoclonal antibody against respiratory syncytial virus (palivizumab) inhibits RSV-induced neurogenic-mediated inflammation in rat airways.Pediatr Res. 2000 Mar;47(3):351-6. doi: 10.1203/00006450-200003000-00011. Pediatr Res. 2000. PMID: 10709734
-
[Respiratory syncytial virus].Uirusu. 2005 Jun;55(1):77-84. doi: 10.2222/jsv.55.77. Uirusu. 2005. PMID: 16308533 Review. Japanese.
-
A review of palivizumab and emerging therapies for respiratory syncytial virus.Expert Opin Biol Ther. 2011 Nov;11(11):1455-67. doi: 10.1517/14712598.2011.608062. Epub 2011 Aug 11. Expert Opin Biol Ther. 2011. PMID: 21831008 Review.
Cited by
-
The Development of Animal Models for Respiratory Syncytial Virus (RSV) Infection and Enhanced RSV Disease.Viruses. 2024 Oct 30;16(11):1701. doi: 10.3390/v16111701. Viruses. 2024. PMID: 39599816 Free PMC article. Review.
References
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

