Gag Virus-like Particles Functionalized with SARS-CoV-2 Variants: Generation, Characterization and Recognition by COVID-19 Convalescent Patients' Sera
- PMID: 38005972
- PMCID: PMC10675557
- DOI: 10.3390/vaccines11111641
Gag Virus-like Particles Functionalized with SARS-CoV-2 Variants: Generation, Characterization and Recognition by COVID-19 Convalescent Patients' Sera
Abstract
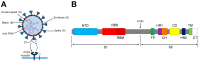
The robustness, safety, versatility, and high immunogenicity of virus-like particles (VLPs) make them a promising approach for the generation of vaccines against a broad range of pathogens. VLPs are recombinant macromolecular structures that closely mimic the native conformation of viruses without carrying viral genetic material. Particularly, HIV-1 Gag-based VLPs are a suitable platform for the presentation of the SARS-CoV-2 Spike (S) protein on their surface. In this context, this work studies the effect of different rationally engineered mutations of the S protein to improve some of its characteristics. The studied variants harbored mutations such as proline substitutions for S stabilization, D614G from the early dominant pandemic form, the elimination of the S1/S2 furin cleavage site to improve S homogeneity, the suppression of a retention motif to favor its membrane localization, and cysteine substitutions to increase its immunogenicity and avoid potential undesired antibody-dependent enhancement (ADE) effects. The influence of the mutations on VLP expression was studied, as well as their immunogenic potential, by testing the recognition of the generated VLP variants by COVID-19 convalescent patients' sera. The results of this work are conceived to give insights on the selection of S protein candidates for their use as immunogens and to showcase the potential of VLPs as carriers for antigen presentation.
Keywords: COVID-19; D614G; SARS-CoV-2; furin cleavage; spike; variants; virus-like particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Optimization, Production, Purification and Characterization of HIV-1 GAG-Based Virus-like Particles Functionalized with SARS-CoV-2.Vaccines (Basel). 2022 Feb 7;10(2):250. doi: 10.3390/vaccines10020250. Vaccines (Basel). 2022. PMID: 35214708 Free PMC article.
-
Production, purification and immunogenicity of Gag virus-like particles carrying SARS-CoV-2 components.Vaccine. 2024 Jan 1;42(1):40-52. doi: 10.1016/j.vaccine.2023.11.048. Epub 2023 Dec 1. Vaccine. 2024. PMID: 38042697
-
Preclinical evaluation of manufacturable SARS-CoV-2 spike virus-like particles produced in Chinese Hamster Ovary cells.Commun Med (Lond). 2023 Aug 23;3(1):116. doi: 10.1038/s43856-023-00340-7. Commun Med (Lond). 2023. PMID: 37612423 Free PMC article.
-
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions.Front Cell Infect Microbiol. 2021 Sep 10;11:690621. doi: 10.3389/fcimb.2021.690621. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568087 Free PMC article. Review.
-
Plant-derived VLP: a worthy platform to produce vaccine against SARS-CoV-2.Biotechnol Lett. 2022 Jan;44(1):45-57. doi: 10.1007/s10529-021-03211-0. Epub 2021 Nov 27. Biotechnol Lett. 2022. PMID: 34837582 Free PMC article. Review.
Cited by
-
Controlling the Quality of Nanodrugs According to Their New Property-Radiothermal Emission.Pharmaceutics. 2024 Jan 26;16(2):180. doi: 10.3390/pharmaceutics16020180. Pharmaceutics. 2024. PMID: 38399241 Free PMC article.
-
Gag HIV-1 Virus-like Particles and Extracellular Vesicles Functionalization with Spike Epitopes of SARS-CoV-2 Using a Copper-Free Click Chemistry Approach.Bioconjug Chem. 2025 Mar 19;36(3):486-499. doi: 10.1021/acs.bioconjchem.4c00559. Epub 2025 Feb 24. Bioconjug Chem. 2025. PMID: 39993141 Free PMC article.
References
-
- Wang C., van Haperen R., Gutiérrez-Álvarez J., Li W., Okba N.M.A., Albulescu I., Widjaja I., van Dieren B., Fernandez-Delgado R., Sola I., et al. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross-reactive monoclonal antibodies. Nat. Commun. 2021;12:1715. doi: 10.1038/s41467-021-21968-w. - DOI - PMC - PubMed
-
- Mohammed I., Nauman A., Paul P., Ganesan S., Chen K.-H., Jalil S.M.S., Jaouni S.H., Kawas H., Khan W.A., Vattoth A.L., et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccin. Immunother. 2022;18:2027160. doi: 10.1080/21645515.2022.2027160. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

