Clinical Utility of SARS-CoV-2 Serological Testing and Defining a Correlate of Protection
- PMID: 38005976
- PMCID: PMC10674881
- DOI: 10.3390/vaccines11111644
Clinical Utility of SARS-CoV-2 Serological Testing and Defining a Correlate of Protection
Abstract
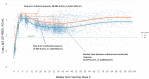
Herein, we review established clinical use cases for SARS-CoV-2 antibody measures, which include diagnosis of recent prior infection, isolating high titer convalescent plasma, diagnosing multisystem inflammatory syndrome in children (MIS-C), and booster dosing in the immunosuppressed and other populations. We then address whether an antibody correlate of protection (CoP) for SARS-CoV-2 has been successfully defined with the following considerations: Antibody responses in the immunocompetent, vaccine type, variants, use of binding antibody tests vs. neutralization tests, and endpoint measures. In the transition from the COVID-19 pandemic to endemic, there has been much interest in defining an antibody CoP. Due to the high mutability of respiratory viruses and our current knowledge of SARS-CoV-2 variants defining a CoP for prevention of infection is unrealistic. However, a CoP may be defined for prevention of severe disease requiring hospitalization and/or death. Most SARS-CoV-2 CoP research has focused on neutralization measurements. However, there can be significant differences in neutralization test methods, and disparate responses to new variants depending on format. Furthermore, neutralization assays are often impractical for high throughput applications (e.g., assessing humoral immune response in populations or large cohorts). Nevertheless, CoP studies using neutralization measures are reviewed to determine where there is consensus. Alternatively, binding antibody tests could be used to define a CoP. Binding antibody assays tend to be highly automatable, high throughput, and therefore practical for large population applications. Again, we review studies for consensus on binding antibody responses to vaccines, focusing on standardized results. Binding antibodies directed against the S1 receptor binding domain (S1-RBD) of the viral spike protein can provide a practical, indirect measure of neutralization. Initially, a response for S1-RBD antibodies may be selected that reflects the peak response in immunocompetent populations and may serve as a target for booster dosing in the immunocompromised. From existing studies reporting peak S1-RBD responses in standardized units, an approximate range of 1372-2744 BAU/mL for mRNA and recombinant protein vaccines was extracted that could serve as an initial CoP target. This target would need to be confirmed and potentially adjusted for updated vaccines, and almost certainly for other vaccine formats (i.e., viral vector). Alternatively, a threshold or response could be defined based on outcomes over time (i.e., prevention of severe disease). We also discuss the precedent for clinical measurement of antibodies for vaccine-preventable diseases (e.g., hepatitis B). Lastly, cellular immunity is briefly addressed for its importance in the nature and durability of protection.
Keywords: SARS-CoV-2; antibodies; clinical utility; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pinto D., Sauer M.M., Czudnochowski N., Low J.S., Tortorici M.A., Housley M.P., Noack J., Walls A.C., Bowen J.E., Guarino B., et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science. 2021;373:1109–1116. doi: 10.1126/science.abj3321. (In English) - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

