Vaccination with an HIV T-Cell Immunogen (HTI) Using DNA Primes Followed by a ChAdOx1-MVA Boost Is Immunogenic in Gut Microbiota-Depleted Mice despite Low IL-22 Serum Levels
- PMID: 38005995
- PMCID: PMC10675013
- DOI: 10.3390/vaccines11111663
Vaccination with an HIV T-Cell Immunogen (HTI) Using DNA Primes Followed by a ChAdOx1-MVA Boost Is Immunogenic in Gut Microbiota-Depleted Mice despite Low IL-22 Serum Levels
Abstract
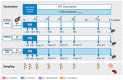
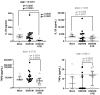
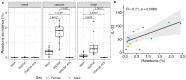
Despite the important role of gut microbiota in the maturation of the immune system, little is known about its impact on the development of T-cell responses to vaccination. Here, we immunized C57BL/6 mice with a prime-boost regimen using DNA plasmid, the Chimpanzee Adenovirus, and the modified Vaccinia Ankara virus expressing a candidate HIV T-cell immunogen and compared the T-cell responses between individuals with an intact or antibiotic-depleted microbiota. Overall, the depletion of the gut microbiota did not result in significant differences in the magnitude or breadth of the immunogen-specific IFNγ T-cell response after vaccination. However, we observed marked changes in the serum levels of four cytokines after vaccinating microbiota-depleted animals, particularly a significant reduction in IL-22 levels. Interestingly, the level of IL-22 in serum correlated with the abundance of Roseburia in the large intestine of mice in the mock and vaccinated groups with intact microbiota. This short-chain fatty acid (SCFA)-producing bacterium was significantly reduced in the vaccinated, microbiota-depleted group. Therefore, our results indicate that, although microbiota depletion reduces serum levels of IL-22, the powerful vaccine regime used could have overcome the impact of microbiota depletion on IFNγ-producing T-cell responses.
Keywords: HIV; IFNγ; IL-22; Roseburia; T-cell; microbiota; vaccine.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. CB is the co-founder, CSO and shareholder of AELIX Therapeutics. All other authors declare no conflict of interest.
Figures

Similar articles
-
Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses.Vaccines (Basel). 2024 Mar 7;12(3):279. doi: 10.3390/vaccines12030279. Vaccines (Basel). 2024. PMID: 38543913 Free PMC article.
-
Vaccination with an HIV T-cell immunogen induces alterations in the mouse gut microbiota.NPJ Biofilms Microbiomes. 2022 Dec 30;8(1):104. doi: 10.1038/s41522-022-00368-y. NPJ Biofilms Microbiomes. 2022. PMID: 36585401 Free PMC article.
-
HIV-1 gp120 and Modified Vaccinia Virus Ankara (MVA) gp140 Boost Immunogens Increase Immunogenicity of a DNA/MVA HIV-1 Vaccine.J Virol. 2017 Nov 30;91(24):e01077-17. doi: 10.1128/JVI.01077-17. Print 2017 Dec 15. J Virol. 2017. PMID: 29021394 Free PMC article.
-
TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia).PLoS Negl Trop Dis. 2011 Jun;5(6):e1204. doi: 10.1371/journal.pntd.0001204. Epub 2011 Jun 14. PLoS Negl Trop Dis. 2011. PMID: 21695103 Free PMC article.
-
A Chimeric HIV-1 gp120 Fused with Vaccinia Virus 14K (A27) Protein as an HIV Immunogen.PLoS One. 2015 Jul 24;10(7):e0133595. doi: 10.1371/journal.pone.0133595. eCollection 2015. PLoS One. 2015. PMID: 26208356 Free PMC article.
Cited by
-
Prospects for therapeutic T-cell vaccine strategies for HIV cure.Curr Opin HIV AIDS. 2025 Sep 1;20(5):463-471. doi: 10.1097/COH.0000000000000965. Epub 2025 Jul 9. Curr Opin HIV AIDS. 2025. PMID: 40638102 Free PMC article. Review.
References
-
- McCoy K.D., Burkhard R., Geuking M.B. The Microbiome and Immune Memory Formation. Immunol. Cell Biol. 2019;97:625–635. - PubMed
-
- Selma-Royo M., Calatayud Arroyo M., García-Mantrana I., Parra-Llorca A., Escuriet R., Martínez-Costa C., Collado M.C. Perinatal Environment Shapes Microbiota Colonization and Infant Growth: Impact on Host Response and Intestinal Function. Microbiome. 2020;8:167. doi: 10.1186/s40168-020-00940-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

