Impact of Bivalent BA.4/5 BNT162b2 COVID-19 Vaccine on Acute Symptoms, Quality of Life, Work Productivity and Activity Levels among Symptomatic US Adults Testing Positive for SARS-CoV-2 at a National Retail Pharmacy
- PMID: 38006001
- PMCID: PMC10675533
- DOI: 10.3390/vaccines11111669
Impact of Bivalent BA.4/5 BNT162b2 COVID-19 Vaccine on Acute Symptoms, Quality of Life, Work Productivity and Activity Levels among Symptomatic US Adults Testing Positive for SARS-CoV-2 at a National Retail Pharmacy
Abstract
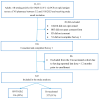
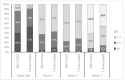
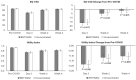
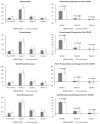
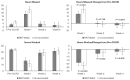
Evidence on the impact of COVID-19 vaccination on symptoms, Health-Related Quality of Life (HRQoL) and Work Productivity and Activity Impairment (WPAI) is scarce. We analyzed associations between bivalent BA.4/5 BNT162b2 (BNT162b2) and these patient-reported outcomes (PROs). Symptomatic US adults testing positive for SARS-CoV-2 were recruited between 2 March and 18 May 2023 (CT.gov NCT05160636). PROs were assessed using four questionnaires measuring symptoms, HRQoL and WPAI (a CDC-based symptom survey, PROMIS Fatigue, EQ-5D-5L, WPAI-GH), from pre-COVID to Week 4 following infection. Multivariable analysis using mixed models for repeated measures was conducted, adjusting for several covariates. The study included 643 participants: 316 vaccinated with BNT162b2 and 327 unvaccinated/not up-to-date. Mean (SD) age was 46.5 years (15.9), 71.2% were female, 44.2% reported prior infection, 25.7% had ≥1 comorbidity. The BNT162b2 cohort reported fewer acute symptoms through Week 4, especially systemic and respiratory symptoms. All PROs were adversely affected, especially at Week 1; however, at that time point, the BNT162b2 cohort reported better work performance, driven by less absenteeism, and fewer work hours lost. No significant differences were observed for HRQoL COVID-19 negatively impacted patient outcomes. Compared with unvaccinated/not up-to-date participants, those vaccinated with bivalent BA.4/5 BNT162b2 reported fewer and less persistent symptoms and improved work performance.
Keywords: BA.4/5 BNT162b2; COVID-19; COVID-19 symptoms; HRQoL; PROMIS Fatigue; SARS-CoV-2; WPAI; bivalent; humanistic; quality of life.
Conflict of interest statement
M.D.F., J.C.C., A.Y., M.B.A., K.E.A., T.M.P., L.P., A.C.S. and S.M.C.L. are employees of Pfizer Inc. and hold stock and/or stock options of Pfizer, Inc. X.S. is an employee of CVS Health and holds stock of CVS Health. L.A.-T. and H.C. were employees of CVS Health when the study was conducted and are now employees of Blue Health Intelligence (BHI), the trade name of Health Intelligence Company, LLC, an independent licensee of the Blue Cross Blue Shield Association.
Figures
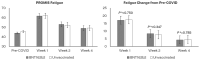
References
-
- Ford N.D., Slaughter D., Edwards D., Dalton A., Perrine C., Vahratian A., Saydah S. Long COVID and Significant Activity Limitation among Adults, by Age—United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023;72:866–870. doi: 10.15585/mmwr.mm7232a3. - DOI - PMC - PubMed
-
- Di Fusco M., Sun X., Moran M.M., Coetzer H., Zamparo J.M., Puzniak L., Alvarez M.B., Tabak Y.P., Cappelleri J.C. Impact of COVID-19 and effects of BNT162b2 on patient-reported outcomes: Quality of life, symptoms, and work productivity among US adult outpatients. J. Patient-Rep. Outcomes. 2022;6:123. doi: 10.1186/s41687-022-00528-w. - DOI - PMC - PubMed
-
- Di Fusco M., Sun X., Moran M.M., Coetzer H., Zamparo J.M., Alvarez M.B., Puzniak L., Tabak Y.P., Cappelleri J.C. Impact of COVID-19 and effects of booster vaccination with BNT162b2 on six-month long COVID symptoms, quality of life, work productivity and activity impairment during Omicron. J. Patient-Rep. Outcomes. 2023;7:77. doi: 10.1186/s41687-023-00616-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

