Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A
- PMID: 38006003
- PMCID: PMC10675568
- DOI: 10.3390/vaccines11111671
Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A
Abstract
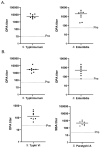
Bloodstream infections in low- and middle-income countries (LMICs) are most frequently attributed to invasive Salmonella disease caused by four primary serovars of Salmonella enterica: Typhi, Paratyphi A, Typhimurium, and Enteritidis. We showed previously that a bivalent vaccine targeting S. Typhi and S. Paratyphi A using a Multiple Antigen-Presenting System (MAPS) induced functional antibodies against S. Typhi and S. Paratyphi. In the current study, we describe the preclinical development of a first candidate quadrivalent combination Salmonella vaccine with the potential to cover all four leading invasive Salmonella serotypes. We showed that the quadrivalent Salmonella MAPS vaccine, containing Vi from S. Typhi, O-specific Polysaccharide (OSP) from S. Paratyphi A, S. Enteritidis and S. Typhimurium, combined with the Salmonella-specific protein SseB, elicits robust and functional antibody responses to each of the components of the vaccine. Our data indicates that the application of MAPS technology to the development of vaccines targeting invasive forms of Salmonella is practical and merits additional consideration.
Keywords: Enteritidis; MAPS vaccine; OSP; Salmonella; SseB; Typhimurium; quadrivalent.
Conflict of interest statement
F.Z., R.M. and Y.-J.L. are inventors of the MAPS technology which is now owned by GSK. F.Z., R.M. and Y.-J.L. are consultants to GSK.
Figures



Similar articles
-
Vi Capsular Polysaccharide Produced by Recombinant Salmonella enterica Serovar Paratyphi A Confers Immunoprotection against Infection by Salmonella enterica Serovar Typhi.Front Cell Infect Microbiol. 2017 Apr 24;7:135. doi: 10.3389/fcimb.2017.00135. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28484685 Free PMC article.
-
A Bivalent MAPS Vaccine Induces Protective Antibody Responses against Salmonella Typhi and Paratyphi A.Vaccines (Basel). 2022 Dec 30;11(1):91. doi: 10.3390/vaccines11010091. Vaccines (Basel). 2022. PMID: 36679935 Free PMC article.
-
A candidate glycoconjugate vaccine induces protective antibodies in the serum and intestinal secretions, antibody recall response and memory T cells and protects against both typhoidal and non-typhoidal Salmonella serovars.Front Immunol. 2024 Jan 9;14:1304170. doi: 10.3389/fimmu.2023.1304170. eCollection 2023. Front Immunol. 2024. PMID: 38264668 Free PMC article.
-
Live attenuated vaccines for invasive Salmonella infections.Vaccine. 2015 Jun 19;33 Suppl 3(0 3):C36-41. doi: 10.1016/j.vaccine.2015.04.029. Epub 2015 Apr 19. Vaccine. 2015. PMID: 25902362 Free PMC article. Review.
-
Glycoconjugate vaccine strategies for protection against invasive Salmonella infections.Hum Vaccin Immunother. 2012 Apr;8(4):494-8. doi: 10.4161/hv.19158. Epub 2012 Feb 28. Hum Vaccin Immunother. 2012. PMID: 22370510 Free PMC article. Review.
Cited by
-
Development of invasive non-typhoidal Salmonella conjugate vaccines and their evaluation in a trivalent formulation with typhoid conjugate vaccine.Vaccine. 2025 Apr 11;52:126913. doi: 10.1016/j.vaccine.2025.126913. Epub 2025 Feb 27. Vaccine. 2025. PMID: 40020336 Free PMC article.
-
Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment.Curr Issues Mol Biol. 2024 Jul 14;46(7):7447-7472. doi: 10.3390/cimb46070442. Curr Issues Mol Biol. 2024. PMID: 39057083 Free PMC article. Review.
-
Foodborne Infections and Salmonella: Current Primary Prevention Tools and Future Perspectives.Vaccines (Basel). 2024 Dec 31;13(1):29. doi: 10.3390/vaccines13010029. Vaccines (Basel). 2024. PMID: 39852807 Free PMC article. Review.
-
Assessing Salmonella Typhi Pathogenicity and Prevention: The Crucial Role of Vaccination in Combating Typhoid Fever.Int J Mol Sci. 2025 Apr 23;26(9):3981. doi: 10.3390/ijms26093981. Int J Mol Sci. 2025. PMID: 40362220 Free PMC article. Review.
-
Consultation report - considerations for a regulatory pathway for bivalent Salmonella Typhi/Paratyphi A vaccines for use in endemic countries.Vaccine. 2025 May 22;56:127189. doi: 10.1016/j.vaccine.2025.127189. Epub 2025 May 1. Vaccine. 2025. PMID: 40318346 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

