Self-Assembling Nanovaccine Fused with Flagellin Enhances Protective Effect against Foot-and-Mouth Disease Virus
- PMID: 38006007
- PMCID: PMC10675102
- DOI: 10.3390/vaccines11111675
Self-Assembling Nanovaccine Fused with Flagellin Enhances Protective Effect against Foot-and-Mouth Disease Virus
Abstract
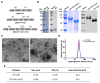
Nanovaccines based on self-assembling nanoparticles (NPs) can show conformational epitopes of antigens and they have high immunogenicity. In addition, flagellin, as a biological immune enhancer, can be fused with an antigen to considerably enhance the immune effect of antigens. In improving the immunogenicity and stability of a foot-and-mouth disease virus (FMDV) antigen, novel FMDV NP antigens were prepared by covalently coupling the VP1 protein and truncated flagellin containing only N-terminus D0 and D1 (N-terminal aa 1-99, nFLiC) with self-assembling NPs (i301). The results showed that the fusion proteins VP1-i301 and VP1-i301-nFLiC can assemble into NPs with high thermal tolerance and stability, obtain high cell uptake efficiency, and upregulate marker molecules and immune-stimulating cytokines in vitro. In addition, compared with monomeric VP1 antigen, high-level cytokines were stimulated with VP1-i301 and VP1-i301-nFLiC nanovaccines in guinea pigs, to provide clinical protection against viral infection comparable to an inactivated vaccine. This study provides new insight for the development of a novel FMD vaccine.
Keywords: flagellin; foot−and−mouth disease; nanoparticles; nanovaccine; self−assembling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Immunogenicity and protective efficacy of recombinant proteins consisting of multiple epitopes of foot-and-mouth disease virus fused with flagellin.Appl Microbiol Biotechnol. 2019 Apr;103(8):3367-3379. doi: 10.1007/s00253-019-09691-5. Epub 2019 Mar 19. Appl Microbiol Biotechnol. 2019. PMID: 30888465
-
Self-Assembling Nanovaccine Enhances Protective Efficacy Against CSFV in Pigs.Front Immunol. 2021 Jul 21;12:689187. doi: 10.3389/fimmu.2021.689187. eCollection 2021. Front Immunol. 2021. PMID: 34367147 Free PMC article.
-
Development of a Subunit Vaccine against Duck Hepatitis A Virus Serotype 3.Vaccines (Basel). 2022 Mar 28;10(4):523. doi: 10.3390/vaccines10040523. Vaccines (Basel). 2022. PMID: 35455272 Free PMC article.
-
A canine adenovirus type 2 vaccine vector confers protection against foot-and-mouth disease in guinea pigs.Vaccine. 2018 Apr 12;36(16):2193-2198. doi: 10.1016/j.vaccine.2018.02.074. Epub 2018 Mar 12. Vaccine. 2018. PMID: 29544690
-
Co-administration of flagellin augments immune responses to inactivated foot-and-mouth disease virus (FMDV) antigen.Res Vet Sci. 2013 Dec;95(3):936-41. doi: 10.1016/j.rvsc.2013.07.021. Epub 2013 Aug 12. Res Vet Sci. 2013. PMID: 23941960
Cited by
-
Co-Delivery of Multiple Toll-Like Receptor Agonists and Avian Influenza Hemagglutinin on Protein Nanoparticles Enhances Vaccine Immunogenicity and Efficacy.Adv Healthc Mater. 2025 Apr;14(10):e2404335. doi: 10.1002/adhm.202404335. Epub 2025 Feb 9. Adv Healthc Mater. 2025. PMID: 39924738 Free PMC article.
-
Structural engineering of flagellin as vaccine adjuvant: quest for the minimal domain of flagellin for TLR5 activation.Mol Biol Rep. 2025 Jan 7;52(1):104. doi: 10.1007/s11033-024-10146-y. Mol Biol Rep. 2025. PMID: 39775323 Free PMC article. Review.
References
-
- Brown F. Foot−and−mouth disease—One of the remaining great plagues. Proc. R. Soc. Lond. B Biol. Sci. 1986;229:215–226. - PubMed
-
- Frenkel H.S. Research on foot−and−mouth disease. III. The cultivation of the virus on a practical scale in explantations of bovine tongue epithelium. Am. J. Vet. Res. 1951;12:187–190. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

