Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®)
- PMID: 38006009
- PMCID: PMC10675389
- DOI: 10.3390/vaccines11111677
Cytokine and Chemokine Production in Mice Inoculated with NVX-CoV2373 (Nuvaxovid®) in Comparison with Omicron BA.4/5 Bivalent BNT162b2 (Comirnaty®)
Abstract
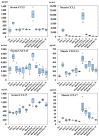
A recombinant SARS-CoV-2 spike protein vaccine (NVX-CoV2373) has been licensed and has a lesser incidence of adverse events. To know the immunological mechanisms of adverse events, the production of cytokines and chemokines was investigated in mice inoculated with NVX-CoV2373. Serum IL-6 was detected on Day 1 of the first and second doses and the IFN-γ, IL-4, IL-10, TNF-α, and IL-6 levels increased on Day 1 of the second dose at the inoculation site. The enhanced production of the inflammatory chemokines (CCL2), homeostatic chemokine (CXCL13), and Th2 chemokine (CCL17) was observed at the inoculation site on Day 1 of the second dose. These findings were compared with data obtained following inoculation with BNT162b2 bivalent vaccine containing omicron BA.4/5. Significantly lower levels of inflammatory chemokines were detected on Day 1 after the first dose of NVX-CoV2373 in sera and inoculation site than those following inoculation with bivalent BNT162b2 (p < 0.01), reflecting a lower incidence of adverse events after immunization with NVX-CoV2373 in humans. NVX-CoV2373 induced significantly higher concentrations of IFN-γ, TNF-α, and IL-10 at the inoculation site obtained on Day 1 of the second dose (p < 0.05). Significant higher levels of Th2 chemokines, CCL11 and CCL17, were induced at the inoculation site on Day 1 of the second dose (p < 0.01) and they explain the booster IgG EIA antibody response after the second dose of NVX-CoV2373.
Keywords: BNT162b2; NVX-CoV2373; adverse events; chemokine; cytokine.
Conflict of interest statement
All authors declare no conflict of interest.
Figures

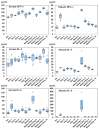

 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05.
: Comirnaty®). * p < 0.05.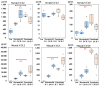
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). ** p < 0.01.
: Comirnaty®). ** p < 0.01.
 : Nuvaxovid®) and orange columns (
: Nuvaxovid®) and orange columns ( : Comirnaty®). * p < 0.05, **
p < 0.01.
: Comirnaty®). * p < 0.05, **
p < 0.01.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

