A Systematic Review and Meta-Analysis of Anti-Rheumatic Drugs and Pneumococcal Vaccine Immunogenicity in Inflammatory Arthritis
- PMID: 38006012
- PMCID: PMC10674424
- DOI: 10.3390/vaccines11111680
A Systematic Review and Meta-Analysis of Anti-Rheumatic Drugs and Pneumococcal Vaccine Immunogenicity in Inflammatory Arthritis
Abstract
Background: Pneumococcal pneumonia is an important cause of morbidity and mortality amongst patients with inflammatory arthritis. Vaccination is recommended by the National Institute for Health and Care Excellence (NICE) but it remains unclear how vaccine efficacy is impacted by different immunosuppressive agents. Our objective was to compare the chance of a seroconversion following vaccination against pneumococcus in patients with inflammatory arthritis to that in the general population, as well as to compare the chance of seroconversion across different targeted therapies.
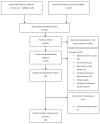
Methods: We searched MEDLINE, Embase and the Cochrane Library databases from inception until 20 June 2023. We included randomized controlled trials and observational studies. Aggregate data were used to undertake a pairwise meta-analysis. Our primary outcome of interest was vaccine seroconversion. We accepted the definition of serological response reported by the authors of each study.
Results: Twenty studies were identified in the systematic review (2807 patients) with ten reporting sufficient data to be included in the meta-analysis (1443 patients). The chance of seroconversion in patients receiving targeted therapies, relative to the general population, was 0.61 (95% CI 0.35 to 1.08). The reduced odds of response were skewed strongly by the effects of abatacept and rituximab with no difference between patients on TNF inhibitors (TNFis) or IL-6 inhibition and healthy controls. Within different inflammatory arthritis populations the findings remained consistent, with rituximab having the strongest negative impact on vaccine response. TNF inhibition monotherapy was associated with a greater chance of vaccine response compared with methotrexate (2.25 (95% CI 1.28 to 3.96)). JAK inhibitor (JAKi) studies were few in number and did not present comparable vaccine response endpoints to include in the meta-analysis. The information available does not suggest any significant detrimental effects of JAKi on vaccine response.
Conclusion: This updated meta-analysis confirms that, for most patients with inflammatory arthritis, pneumococcal vaccine can be administered with confidence and that it will achieve comparable seroconversion rates to the healthy population. Patients on rituximab were the group least likely to achieve a response and further research is needed to explore the value of multiple-course pneumococcal vaccination schedules in this population.
Keywords: immunosuppression; pneumococcal vaccination; rheumatoid arthritis.
Conflict of interest statement
DN receives honoraria from AbbVie and Galapagos. JBG has received speaker fees from AbbVie, Galapagos, Janssen, Lilly, Novartis, Pfizer and UCB. MDR has received speaker fees and educational grants from Janssen, Lilly, Menarini, Pfizer and UCB. APC has received grant funding from BMS, and consulting fees from BMS, AbbVie and GSK/Galvani. The remaining authors declare no conflicts of interest relevant to this article.
Figures



References
-
- Bass A.R., Chakravarty E., Akl E.A., Bingham C.O., Calabrese L., Cappelli L.C., Johnson S.R., Imundo L.F., Winthrop K.L., Arasaratnam R.J., et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients With Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2023;75:333–348. doi: 10.1002/art.42386. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

