Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines
- PMID: 38006022
- PMCID: PMC10675184
- DOI: 10.3390/vaccines11111690
Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines
Abstract
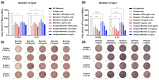
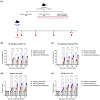
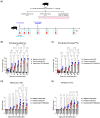
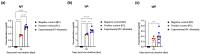
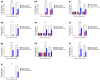
An inactivated whole-virus vaccine is currently used to prevent foot-and-mouth disease (FMD). Although this vaccine is effective, it offers short-term immunity that requires regular booster immunizations and has several side effects, including local reactions at the vaccination site. To address these limitations, herein, we evaluated the efficacy of bestatin as a novel small molecule adjuvant for inactivated FMD vaccines. Our findings showed that the FMD vaccine formulated with bestatin enhanced early, intermediate-, and particularly long-term immunity in experimental animals (mice) and target animals (pigs). Furthermore, cytokines (interferon (IFN)α, IFNβ, IFNγ, and interleukin (IL)-29), retinoic acid-inducible gene (RIG)-I, and T-cell and B-cell core receptors (cluster of differentiation (CD)28, CD19, CD21, and CD81) markedly increased in the group that received the FMD vaccine adjuvanted with bestatin in pigs compared with the control. These results indicate the significant potential of bestatin to improve the efficacy of inactivated FMD vaccines in terms of immunomodulatory function for the simultaneous induction of potent cellular and humoral immune response and a long-lasting memory response.
Keywords: adjuvant; bestatin; cellular and humoral immunity; foot-and-mouth disease; inactivated vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hwang J.H., Lee G., Kim A., Park J.H., Lee M.J., Kim B., Kim S.M. A vaccine strain of the A/ASIA/Sea-97 lineage of foot-and-mouth disease virus with a single amino acid substitution in the P1 region that is adapted to suspension culture provides high immunogenicity. Vaccines. 2021;9:308. doi: 10.3390/vaccines9040308. - DOI - PMC - PubMed
-
- Jo H., Kim B.Y., Park S.H., Kim H.M., Shin S.H., Hwang S.Y., Kim S.M., Kim B., Park J.H., Lee M.J. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. npj Vaccines. 2021;6:42. doi: 10.1038/s41541-021-00304-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

