A Subunit Vaccine Based on the VP2 Protein of Porcine Parvovirus 1 Induces a Strong Protective Effect in Pregnant Gilts
- PMID: 38006024
- PMCID: PMC10675385
- DOI: 10.3390/vaccines11111692
A Subunit Vaccine Based on the VP2 Protein of Porcine Parvovirus 1 Induces a Strong Protective Effect in Pregnant Gilts
Abstract
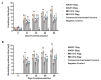
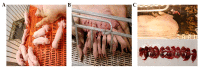
Porcine parvovirus 1 (PPV1) is one of the most prevalent pathogens that can cause reproductive disorder in sows. The VP2 protein of PPV1 is the most important immunogenic protein that induces neutralizing antibodies and protective immunity. Thus, VP2 is considered an ideal target antigen for the development of a genetically engineered PPV1 vaccine. In this study, the baculovirus transfer vector carrying the HR5-P10-VP2 expression cassette was successfully constructed with the aim of increasing the expression levels of the VP2 protein. The VP2 protein was confirmed using SDS‒PAGE and Western blot analyses. Electronic microscope analysis showed that the recombinant VP2 proteins were capable of self-assembling into VLPs with a diameter of approximately 25 nm. The immunogenicity of the VP2 subunit vaccine was evaluated in pigs. The results showed that VP2 protein emulsified with ISA 201VG adjuvant induced higher levels of HI antibodies and neutralizing antibodies than VP2 protein emulsified with IMS 1313VG adjuvant. Furthermore, the gilts immunized with the ISA 201VG 20 μg subunit vaccine acquired complete protection against PPV1 HN2019 infection. In contrast, the commercial inactivated vaccine provided incomplete protection in gilts. Therefore, the VP2 subunit vaccine is a promising genetically engineered vaccine for the prevention and control of PPV1.
Keywords: VP2 protein; porcine parvovirus 1; protective immunity; subunit vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Plant-Produced Porcine Parvovirus 1-82 VP2 Subunit Vaccine Protects Pregnant Sows against Challenge with a Genetically Heterologous PPV1 Strain.Vaccines (Basel). 2022 Dec 26;11(1):54. doi: 10.3390/vaccines11010054. Vaccines (Basel). 2022. PMID: 36679898 Free PMC article.
-
The immunogenicity of the virus-like particles derived from the VP2 protein of porcine parvovirus.Vet Microbiol. 2020 Sep;248:108795. doi: 10.1016/j.vetmic.2020.108795. Epub 2020 Jul 10. Vet Microbiol. 2020. PMID: 32827923
-
Duration of immunity against heterologous porcine parvovirus 1 challenge in gilts immunized with a novel subunit vaccine based on the viral protein 2.BMC Vet Res. 2020 Jun 9;16(1):184. doi: 10.1186/s12917-020-02394-4. BMC Vet Res. 2020. PMID: 32517691 Free PMC article.
-
Effects of three commercial vaccines against porcine parvovirus 1 in pregnant gilts.Vaccine. 2021 Jun 29;39(29):3997-4005. doi: 10.1016/j.vaccine.2021.05.042. Epub 2021 Jun 5. Vaccine. 2021. PMID: 34099327
-
Large-scale manufacture of VP2 VLP vaccine against porcine parvovirus in Escherichia coli with high-density fermentation.Appl Microbiol Biotechnol. 2020 May;104(9):3847-3857. doi: 10.1007/s00253-020-10483-5. Epub 2020 Mar 4. Appl Microbiol Biotechnol. 2020. PMID: 32130468
Cited by
-
Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2.Vaccines (Basel). 2025 Jul 8;13(7):736. doi: 10.3390/vaccines13070736. Vaccines (Basel). 2025. PMID: 40733713 Free PMC article.
-
The Identification of Dual T-Cell and B-Cell Epitopes Within Viral Proteins Utilizing a Comprehensive Peptide Array Approach.Vaccines (Basel). 2025 Feb 26;13(3):239. doi: 10.3390/vaccines13030239. Vaccines (Basel). 2025. PMID: 40266119 Free PMC article.
References
-
- Cho K.-N., Ouh I.-O., Park Y.-M., Park M.-H., Min K.-M., Kang H.-J., Yun S.-Y., Song J.-Y., Hyun B.-H., Park C.-K., et al. A Plant-Produced Porcine Parvovirus 1-82 VP2 Subunit Vaccine Protects Pregnant Sows against Challenge with a Genetically Heterologous PPV1 Strain. Vaccines. 2023;11:54. doi: 10.3390/vaccines11010054. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

