Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays
- PMID: 38006026
- PMCID: PMC10674746
- DOI: 10.3390/vaccines11111694
Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays
Abstract
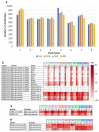
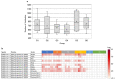
Although studies have demonstrated that infections with various viruses, bacteria, and parasites can modulate the immune system, no study has investigated changes in antibodies against microbial antigens after the COVID-19 mRNA vaccination. IgG antibodies against microbial antigens in the blood of vaccinees were comprehensively analyzed using microbial protein microarrays that carried approximately 5000 microbe-derived proteins. Changes in antibodies against microbial antigens were scrutinized in healthy participants enrolled in the Fukushima Vaccination Community Survey conducted in Fukushima Prefecture, Japan, after their second and third COVID-19 mRNA vaccinations. Antibody profiling of six groups stratified by antibody titer and the remaining neutralizing antibodies was also performed to study the dynamics of neutralizing antibodies against SARS-CoV-2 and the changes in antibodies against microbial antigens. The results showed that changes in antibodies against microbial antigens other than SARS-CoV-2 antigens were extremely limited after COVID-19 vaccination. In addition, antibodies against a staphylococcal complement inhibitor have been identified as microbial antigens that are associated with increased levels of neutralizing antibodies against SARS-CoV-2. These antibodies may be a predictor of the maintenance of neutralizing antibodies following the administration of a COVID-19 mRNA vaccine.
Keywords: COVID-19; antibody; bacteria; vaccine.
Conflict of interest statement
Yudai Kaneko is employed by the company Medical & Biological Laboratories, Co., Ltd., Tokyo, Japan. MBL imported the testing material used in this research. Yurie Kobashi and Masaharu Tsubokura received a research grant from the Pfizer Health Research Foundation for research not associated with this work. Masaharu Tsubokura received a research grant from Moderna Inc. outside of this work. The remaining authors declare no potential conflicts of interest.
Figures


Similar articles
-
Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2.PLoS One. 2022 Oct 18;17(10):e0263861. doi: 10.1371/journal.pone.0263861. eCollection 2022. PLoS One. 2022. PMID: 36256664 Free PMC article.
-
Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine.PLoS One. 2022 Jun 10;17(6):e0269917. doi: 10.1371/journal.pone.0269917. eCollection 2022. PLoS One. 2022. PMID: 35687563 Free PMC article.
-
Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine.JAMA Netw Open. 2022 May 2;5(5):e2210780. doi: 10.1001/jamanetworkopen.2022.10780. JAMA Netw Open. 2022. PMID: 35532938 Free PMC article.
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
Correlation of SARS-CoV-2 Viral Neutralizing Antibody Titers with Anti-Spike Antibodies and ACE-2 Inhibition among Vaccinated Individuals.Microbiol Spectr. 2022 Oct 26;10(5):e0131522. doi: 10.1128/spectrum.01315-22. Epub 2022 Sep 19. Microbiol Spectr. 2022. PMID: 36121252 Free PMC article.
Cited by
-
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis.Front Public Health. 2024 Jul 30;12:1406315. doi: 10.3389/fpubh.2024.1406315. eCollection 2024. Front Public Health. 2024. PMID: 39139673 Free PMC article.
-
A New Vaccine Candidate Expressing JUNV GP1-GP2 Against Argentine Hemorrhagic Fever Based on Baculovirus Surface Display.Curr Microbiol. 2025 Jun 13;82(8):334. doi: 10.1007/s00284-025-04320-z. Curr Microbiol. 2025. PMID: 40514553
References
-
- Coronavirus Disease (COVID-19) Pandemic. [(accessed on 5 September 2023)]. Available online: https://www.who.int/europe/emergencies/situations/covid-19.
-
- COVID-19 Vaccines. [(accessed on 5 September 2023)]. Available online: https://japan.kantei.go.jp/ongoingtopics/vaccine.html.
-
- Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., Groome M.J., Huppert A., O’Brien K.L., Smith P.G., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

