Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study
- PMID: 38006035
- PMCID: PMC10675794
- DOI: 10.3390/vaccines11111703
Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study
Abstract
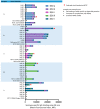
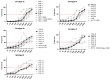
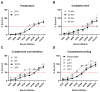
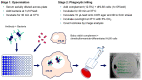
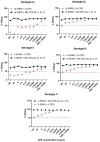
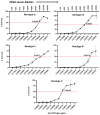
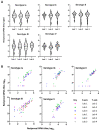
The placental transfer of antibodies that mediate bacterial clearance via phagocytes is likely important for protection against invasive group B Streptococcus (GBS) disease. A robust functional assay is essential to determine the immune correlates of protection and assist vaccine development. Using standard reagents, we developed and optimized an opsonophagocytic killing assay (OPKA) where dilutions of test sera were incubated with bacteria, baby rabbit complement (BRC) and differentiated HL60 cells (dHL60) for 30 min. Following overnight incubation, the surviving bacteria were enumerated and the % bacterial survival was calculated relative to serum-negative controls. A reciprocal 50% killing titer was then assigned. The minimal concentrations of anti-capsular polysaccharide (CPS) IgG required for 50% killing were 1.65-3.70 ng/mL (depending on serotype). Inhibition of killing was observed using sera absorbed with homologous CPS but not heterologous CPS, indicating specificity for anti-CPS IgG. The assay performance was examined in an interlaboratory study using residual sera from CPS-conjugate vaccine trials with international partners in the Group B Streptococcus Assay STandardisatiON (GASTON) Consortium. Strong correlations of reported titers between laboratories were observed: ST-Ia r = 0.88, ST-Ib r = 0.91, ST-II r = 0.91, ST-III r = 0.90 and ST-V r = 0.94. The OPKA is an easily transferable assay with accessible standard reagents and will be a valuable tool to assess GBS-specific antibodies in natural immunity and vaccine studies.
Keywords: Group B Streptococcus; OPKA; correlates of protection; neonatal antibodies; opsonophagocytosis assay; transplacental antibodies; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Vekemans J., Crofts J., Baker C.J., Goldblatt D., Heath P.T., Madhi S.A., Le Doare K., Andrews N., Pollard A.J., Saha S.K., et al. The Role of Immune Correlates of Protection on the Pathway to Licensure, Policy Decision and Use of Group B Streptococcus Vaccines for Maternal Immunization: Considerations from World Health Organization Consultations. Vaccine. 2019;37:3190–3198. doi: 10.1016/j.vaccine.2019.04.039. - DOI - PMC - PubMed
-
- Absalon J., Segall N., Block S.L., Center K.J., Scully I.L., Giardina P.C., Peterson J., Watson W.J., Gruber W.C., Jansen K.U., et al. Safety and Immunogenicity of a Novel Hexavalent Group B Streptococcus Conjugate Vaccine in Healthy, Non-Pregnant Adults: A Phase 1/2, Randomised, Placebo-Controlled, Observer-Blinded, Dose-Escalation Trial. Lancet Infect. Dis. 2021;21:263–274. doi: 10.1016/S1473-3099(20)30478-3. - DOI - PMC - PubMed
-
- Gonzalez-Miro M., Pawlowski A., Lehtonen J., Cao D., Larsson S., Darsley M., Kitson G., Fischer P.B., Johansson-Lindbom B. Safety and Immunogenicity of the Group B Streptococcus Vaccine AlpN in a Placebo-Controlled Double-Blind Phase 1 Trial. iScience. 2023;26:106261. doi: 10.1016/j.isci.2023.106261. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

