Primary and Recall Immune Responses to SARS-CoV-2 in Breakthrough Infection
- PMID: 38006037
- PMCID: PMC10675240
- DOI: 10.3390/vaccines11111705
Primary and Recall Immune Responses to SARS-CoV-2 in Breakthrough Infection
Abstract
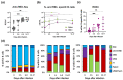
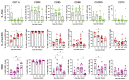
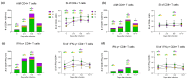
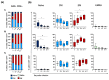
Breakthrough infections in SARS-CoV-2 vaccinated individuals are an ideal circumstance for the simultaneous exploration of both the vaccine-induced memory reaction to the spike (S) protein and the primary response to the membrane (M) and nucleocapsid (N) proteins generated by natural infection. We monitored 15 healthcare workers who had been vaccinated with two doses of Pfizer BioNTech BNT162b2 and were then later infected with the SARS-CoV-2 B.1.617.2. (Delta) variant, analysing the antiviral humoral and cellular immune responses. Natural infection determined an immediate and sharp rise in anti-RBD antibody titres and in the frequency of both S-specific antibody secreting cells (ASCs) and memory B lymphocytes. T cells responded promptly to infection by activating and expanding already at 2-5 days. S-specific memory and emerging M- and N-specific T cells both expressed high levels of activation markers and showed effector capacity with similar kinetics but with different magnitude. The results show that natural infection with SARS-CoV-2 in vaccinated individuals induces fully functional and rapidly expanding T and B lymphocytes in concert with the emergence of novel virus-specific T cells. This swift and punctual response also covers viral variants and captures a paradigmatic case of a healthy adaptive immune reaction to infection with a mutating virus.
Keywords: SARS-CoV-2; adaptive immune response; breakthrough infection; vaccination.
Conflict of interest statement
The authors report no conflict of interest.
Figures
Similar articles
-
Humoral and cellular immune responses in fully vaccinated individuals with or without SARS-CoV-2 breakthrough infection: Results from the CoV-ADAPT cohort.J Med Virol. 2023 Oct;95(10):e29122. doi: 10.1002/jmv.29122. J Med Virol. 2023. PMID: 37787583
-
Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT - IRCCS "Fondazione Pascale" Cancer Center (Naples, Italy).Infect Agent Cancer. 2022 Jul 28;17(1):40. doi: 10.1186/s13027-022-00451-1. Infect Agent Cancer. 2022. PMID: 35902961 Free PMC article.
-
Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: an open-label, observational phase 4 trial.Lancet Microbe. 2023 Aug;4(8):e612-e621. doi: 10.1016/S2666-5247(23)00107-6. Epub 2023 Jun 21. Lancet Microbe. 2023. PMID: 37354911 Free PMC article. Clinical Trial.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
[Investigation of SARS-CoV-2-Specific Humoral and Cellular Immunity Values in Health Care Workers with COVID-19 Disease and Administered with COVID-19 Vaccine].Mikrobiyol Bul. 2022 Jul;56(3):480-492. doi: 10.5578/mb.20229708. Mikrobiyol Bul. 2022. PMID: 35960239 Turkish.
Cited by
-
Monitoring of adaptive immune responses in healthcare workers who received a Coronavirus disease 2019 vaccine booster dose.Health Sci Rep. 2024 Jul 16;7(7):e2250. doi: 10.1002/hsr2.2250. eCollection 2024 Jul. Health Sci Rep. 2024. PMID: 39015422 Free PMC article.
References
-
- Rahmani K., Shavaleh R., Forouhi M., Disfani H.F., Kamandi M., Oskooi R.K., Foogerdi M., Soltani M., Rahchamani M., Mohaddespour M., et al. The Effectiveness of COVID-19 Vaccines in Reducing the Incidence, Hospitalization, and Mortality from COVID-19: A Systematic Review and Meta-Analysis. Front. Public Health. 2022;10:873596. doi: 10.3389/fpubh.2022.873596. - DOI - PMC - PubMed
-
- Yang Z.-R., Jiang Y.-W., Li F.-X., Liu D., Lin T.-F., Zhao Z.-Y., Wei C., Jin Q.-Y., Li X.-M., Jia Y.-X., et al. Efficacy of SARS-CoV-2 Vaccines and the Dose–Response Relationship with Three Major Antibodies: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet Microbe. 2023;4:e236–e246. doi: 10.1016/S2666-5247(22)00390-1. - DOI - PMC - PubMed
-
- Andeweg S.P., de Gier B., Eggink D., van den Ende C., van Maarseveen N., Ali L., Vlaemynck B., Schepers R., Hahné S.J.M., Reusken C.B.E.M., et al. Protection of COVID-19 Vaccination and Previous Infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 Infections. Nat. Commun. 2022;13:4738. doi: 10.1038/s41467-022-31838-8. - DOI - PMC - PubMed
-
- Fiolet T., Kherabi Y., MacDonald C.-J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

