Efficacy of Fowlpox Virus Vector Vaccine Expressing VP2 and Chicken Interleukin-18 in the Protection against Infectious Bursal Disease Virus
- PMID: 38006048
- PMCID: PMC10675466
- DOI: 10.3390/vaccines11111716
Efficacy of Fowlpox Virus Vector Vaccine Expressing VP2 and Chicken Interleukin-18 in the Protection against Infectious Bursal Disease Virus
Abstract
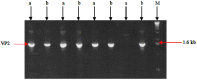
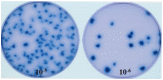
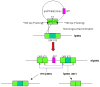
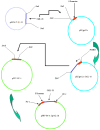
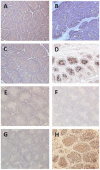
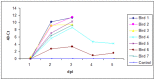
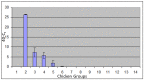
In mammals, the role of interleukin-18 (IL-18) in the immune response is to drive inflammatory and, normally therefore, anti-viral responses. IL-18 also shows promise as a vaccine adjuvant in mammals. Chicken IL-18 (chIL-18) has been cloned. The aim of this study was to investigate the potential of chIL-18 to act as a vaccine adjuvant in the context of a live recombinant Fowlpox virus vaccine (fpIBD1) against Infectious bursal disease virus (IBDV). fpIBD1 protects against mortality, but not against damage to the bursa of Fabricius caused by IBDV infection. The Fowlpox virus genome itself contains several candidate immunomodulatory genes, including potential IL-18 binding proteins (IL-18bp). We knocked out (Δ) the potential IL-18bp genes in fpIBD1 and inserted (::) the cDNA encoding chIL-18 into fpIBD1 in the non-essential ORF030, generating five new viral constructs -fpIBD1::chIL-18, fpIBD1ΔORF073, fpIBD1ΔORF073::chIL-18, fpIBD1ΔORF214, and fpIBD1ΔORF214::chIL-18. The subsequent protection from challenge with virulent IBDV, as measured by viral load and bursal damage, given by these altered fpIBD1 strains, was compared to that given by the original fpIBD1. Complete protection was provided following challenge with IBDV in chicken groups vaccinated with either fpIBDIΔ073::IL-18 or fpIBD1Δ214::IL-18, as no bursal damage nor IBDV was detected in the bursae of the birds. The results show that chIL-18 can act as an effective vaccine adjuvant by improving the fpIBD1 vaccine and providing complete protection against IBDV challenge.
Keywords: chicken interleukin-18; fpIBD1; recombinant Fowlpox virus FP9; vaccine adjuvant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
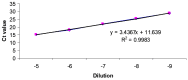

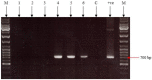
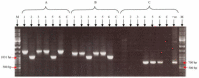
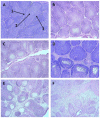
Similar articles
-
Protection from IBDV-induced bursal damage by a recombinant fowlpox vaccine, fpIBD1, is dependent on the titre of challenge virus and chicken genotype.Vaccine. 2000 Aug 1;18(28):3230-41. doi: 10.1016/s0264-410x(00)00133-x. Vaccine. 2000. PMID: 10869768
-
Enhancement of the immunogenicity of DNA vaccine against infectious bursal disease virus by co-delivery with plasmid encoding chicken interleukin 2.Virology. 2004 Nov 10;329(1):89-100. doi: 10.1016/j.virol.2004.07.033. Virology. 2004. PMID: 15476877
-
Mucosal application of cationic poly(D,L-lactide-co-glycolide) microparticles as carriers of DNA vaccine and adjuvants to protect chickens against infectious bursal disease.Vaccine. 2013 Aug 12;31(36):3656-62. doi: 10.1016/j.vaccine.2013.06.011. Epub 2013 Jun 15. Vaccine. 2013. PMID: 23777953
-
Chicken IL-7 as a potent adjuvant enhances IBDV VP2 DNA vaccine immunogenicity and protective efficacy.Vet Microbiol. 2016 Sep 25;193:145-55. doi: 10.1016/j.vetmic.2016.08.016. Epub 2016 Aug 17. Vet Microbiol. 2016. PMID: 27599941
-
Co-Expression of Chicken IL-2 and IL-7 Enhances the Immunogenicity and Protective Efficacy of a VP2-Expressing DNA Vaccine against IBDV in Chickens.Viruses. 2019 May 24;11(5):476. doi: 10.3390/v11050476. Viruses. 2019. PMID: 31137731 Free PMC article.
Cited by
-
Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein.Vaccines (Basel). 2025 Jul 16;13(7):758. doi: 10.3390/vaccines13070758. Vaccines (Basel). 2025. PMID: 40733736 Free PMC article.
-
Current Status of Poultry Recombinant Virus Vector Vaccine Development.Vaccines (Basel). 2024 Jun 6;12(6):630. doi: 10.3390/vaccines12060630. Vaccines (Basel). 2024. PMID: 38932359 Free PMC article. Review.
-
Revolutionizing Veterinary Health with Viral Vector-Based Vaccines.Indian J Microbiol. 2024 Sep;64(3):867-878. doi: 10.1007/s12088-024-01341-3. Epub 2024 Jun 21. Indian J Microbiol. 2024. PMID: 39282171 Review.
-
Development and evaluation of immunogenicity and protective efficacy of two recombinant attenuated newcastle disease viruses expressing the VP2 protein of infectious bursal disease virus.Poult Sci. 2025 Jul;104(7):105253. doi: 10.1016/j.psj.2025.105253. Epub 2025 May 8. Poult Sci. 2025. PMID: 40339237 Free PMC article.
References
-
- Cosgrove A. An Apparently New Disease of Chickens: Avian Nephrosis. Avian Dis. 1962;6:385. doi: 10.2307/1587909. - DOI
-
- Eterradossi N., Saif Y.M. Infectious bursal disease. In: Swayne D.E., Boulianne M., Logue C.M., McDougald L.R., Nair V., Suarez D.L., de Wit S., Grimes T., Johnson D., Kromm M., et al., editors. Diseases of Poultry. 14th ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2020. pp. 257–283.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

