Immunotherapeutic Agents for Intratumoral Immunotherapy
- PMID: 38006049
- PMCID: PMC10674963
- DOI: 10.3390/vaccines11111717
Immunotherapeutic Agents for Intratumoral Immunotherapy
Abstract
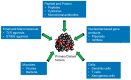
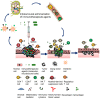
Immunotherapy using systemic immune checkpoint inhibitors (ICI) and chimeric antigen receptor (CAR) T cells has revolutionized cancer treatment, but it only benefits a subset of patients. Systemic immunotherapies cause severe autoimmune toxicities and cytokine storms. Immune-related adverse events (irAEs) plus the immunosuppressive tumor microenvironment (TME) have been linked to the inefficacy of systemic immunotherapy. Intratumoral immunotherapy that increases immunotherapeutic agent bioavailability inside tumors could enhance the efficacy of immunotherapies and reduce systemic toxicities. In preclinical and clinical studies, intratumoral administration of immunostimulatory agents from small molecules to xenogeneic cells has demonstrated antitumor effects not only on the injected tumors but also against noninjected lesions. Herein, we review and discuss the results of these approaches in preclinical models and clinical trials to build the landscape of intratumoral immunotherapeutic agents and we describe how they stimulate the body's immune system to trigger antitumor immunity as well as the challenges in clinical practice. Systemic and intratumoral combination immunotherapy would make the best use of the body's immune system to treat cancers. Combining precision medicine and immunotherapy in cancer treatment would treat both the mutated targets in tumors and the weakened body's immune system simultaneously, exerting maximum effects of the medical intervention.
Keywords: antitumor immunity; body; immunotherapeutic; intratumoral; neoantigen; xenoantigen.
Conflict of interest statement
Chih-Rong Shyr and Chi-Ping Huang are inventors on issued and pending patent applications in the areas described in this review. Chih-Rong Shyr is an employee of eXCELL Biotherapeutics. Lang-Chi Liu, Chih-Rong Shyr and Chi-Ping Huang hold equity in eXCELL Biotherapeutics. Hui-Shan Chien has nothing to disclose.
Figures
References
-
- Ambrus J.L., Ambrus C.M., Mink I.B., Pickren J.W. Causes of death in cancer patients. J. Med. 1975;6:61–64. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

