Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine
- PMID: 38006052
- PMCID: PMC10675463
- DOI: 10.3390/vaccines11111720
Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine
Abstract
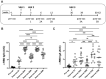
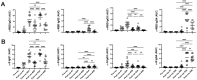
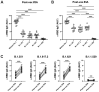
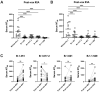
SARS-CoV-2 mRNA vaccines are administered as effective prophylactic measures for reducing virus transmission rates and disease severity. To enhance the durability of post-vaccination immunity and combat SARS-CoV-2 variants, boosters have been administered to two-dose vaccinees. However, long-term humoral responses following booster vaccination are not well characterized. A 16-member cohort of healthy SARS-CoV-2 naïve participants were enrolled in this study during a three-dose BNT162b2 vaccine series. Serum samples were collected from vaccinees over 420 days and screened for antigen (Ag)-specific antibody titers, IgG subclass distribution, and neutralizing antibody (nAb) responses. Vaccine boosting restored peak Ag-specific titers with sustained α-RBD IgG and IgA antibody responses when measured at six months post-boost. RBD- and spike-specific IgG4 antibody levels were markedly elevated in three-dose but not two-dose immune sera. Although strong neutralization responses were detected in two- and three-dose vaccine sera, these rapidly decayed to pre-immune levels by four and six months, respectively. While boosters enhanced serum IgG Ab reactivity and nAb responses against variant strains, all variants tested showed resistance to two- and three-dose immune sera. Our data reflect the poor durability of vaccine-induced nAb responses which are a strong predictor of protection from symptomatic SARS-CoV-2 infection. The induction of IgG4-switched humoral responses may permit extended viral persistence via the downregulation of Fc-mediated effector functions.
Keywords: BNT162b2 mRNA vaccine; IgG4 subclass response; SARS-CoV-2; neutralizing antibodies; serum IgG and IgA kinetics; spike (S) and receptor-binding domain (RBD).
Conflict of interest statement
The authors declare no conflict of interest and certify that this research was conducted independently of any commercial or financial relationships.
Figures

Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2.PLoS One. 2022 Oct 18;17(10):e0263861. doi: 10.1371/journal.pone.0263861. eCollection 2022. PLoS One. 2022. PMID: 36256664 Free PMC article.
-
Hybrid Immunity Shifts the Fc-Effector Quality of SARS-CoV-2 mRNA Vaccine-Induced Immunity.mBio. 2022 Oct 26;13(5):e0164722. doi: 10.1128/mbio.01647-22. Epub 2022 Aug 24. mBio. 2022. PMID: 36000735 Free PMC article.
-
Correlation between anti-S IgG and neutralizing antibody titers against three live SARS-CoV-2 variants in BNT162b2 vaccine recipients.Hum Vaccin Immunother. 2022 Nov 30;18(6):2105611. doi: 10.1080/21645515.2022.2105611. Epub 2022 Sep 12. Hum Vaccin Immunother. 2022. PMID: 36094467 Free PMC article.
-
SARS-CoV-2 Infection-and mRNA Vaccine-induced Humoral Immunity among Schoolchildren in Hawassa, Ethiopia.Front Immunol. 2023 Jun 15;14:1163688. doi: 10.3389/fimmu.2023.1163688. eCollection 2023. Front Immunol. 2023. PMID: 37398668 Free PMC article.
Cited by
-
Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort.Pathogens. 2025 Jan 8;14(1):44. doi: 10.3390/pathogens14010044. Pathogens. 2025. PMID: 39861005 Free PMC article.
-
Immunosuppressive Therapy Modifies Anti-Spike IgG Subclasses Distribution After Four Doses of mRNA Vaccination in a Cohort of Kidney Transplant Recipients.Vaccines (Basel). 2025 Jan 25;13(2):123. doi: 10.3390/vaccines13020123. Vaccines (Basel). 2025. PMID: 40006670 Free PMC article.
-
Diversified humoral immunity and impacts of booster vaccines: SARS-CoV-2 antibody profile and Omicron BA.2 neutralization before and after first or second boosters.Microbiol Spectr. 2024 Oct 3;12(10):e0060524. doi: 10.1128/spectrum.00605-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162540 Free PMC article.
-
Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review.Int J Mol Sci. 2025 Jan 20;26(2):862. doi: 10.3390/ijms26020862. Int J Mol Sci. 2025. PMID: 39859575 Free PMC article. Review.
-
Avidity maturation of anti-spike IgG after vaccination in COVID-19 convalescent vs COVID-19 naïve patients.APMIS. 2025 Jan;133(1):e13489. doi: 10.1111/apm.13489. Epub 2024 Nov 7. APMIS. 2025. PMID: 39509082 Free PMC article.
References
-
- Savinkina A., Bilinski A., Fitzpatrick M., Paltiel A.D., Rizvi Z., Salomon J., Thornhill T., Gonsalves G. Estimating deaths averted and cost per life saved by scaling up mRNA COVID-19 vaccination in low-income and lower-middle-income countries in the COVID-19 Omicron variant era: A modelling study. BMJ Open. 2022;12:e061752. doi: 10.1136/bmjopen-2022-061752. - DOI - PMC - PubMed
-
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

