CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond
- PMID: 38006053
- PMCID: PMC10674477
- DOI: 10.3390/vaccines11111721
CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond
Abstract
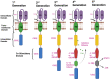
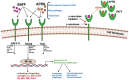
Significant progress has been achieved in the realm of therapeutic interventions for multiple myeloma (MM), leading to transformative shifts in its clinical management. While conventional modalities such as surgery, radiotherapy, and chemotherapy have improved the clinical outcomes, the overarching challenge of effecting a comprehensive cure for patients afflicted with relapsed and refractory MM (RRMM) endures. Notably, adoptive cellular therapy, especially chimeric antigen receptor T-cell (CAR-T) therapy, has exhibited efficacy in patients with refractory or resistant B-cell malignancies and is now also being tested in patients with MM. Within this context, the B-cell maturation antigen (BCMA) has emerged as a promising candidate for CAR-T-cell antigen targeting in MM. Alternative targets include SLAMF7, CD38, CD19, the signaling lymphocyte activation molecule CS1, NKG2D, and CD138. Numerous clinical studies have demonstrated the clinical efficacy of these CAR-T-cell therapies, although longitudinal follow-up reveals some degree of antigenic escape. The widespread implementation of CAR-T-cell therapy is encumbered by several barriers, including antigenic evasion, uneven intratumoral infiltration in solid cancers, cytokine release syndrome, neurotoxicity, logistical implementation, and financial burden. This article provides an overview of CAR-T-cell therapy in MM and the utilization of BCMA as the target antigen, as well as an overview of other potential target moieties.
Keywords: B-cell maturation antigen (BCMA); CAR-T-cell therapy; multiple myeloma (MM).
Conflict of interest statement
Kamal S. Saini reports consulting fees from the European Commission and stock and/or other ownership interests in Labcorp Inc., Fortrea Inc., and Quantum Health Analytics (UK) Ltd., outside the submitted work. The other authors do not report any conflict of interest.
Figures

Similar articles
-
A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma.J Hematol Oncol. 2021 Oct 9;14(1):161. doi: 10.1186/s13045-021-01170-7. J Hematol Oncol. 2021. PMID: 34627333 Free PMC article. Clinical Trial.
-
Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy.Front Immunol. 2018 Aug 10;9:1821. doi: 10.3389/fimmu.2018.01821. eCollection 2018. Front Immunol. 2018. PMID: 30147690 Free PMC article. Review.
-
Beyond BCMA: the next wave of CAR T cell therapy in multiple myeloma.Front Oncol. 2024 May 10;14:1398902. doi: 10.3389/fonc.2024.1398902. eCollection 2024. Front Oncol. 2024. PMID: 38800372 Free PMC article. Review.
-
CAR T Cells and Other Cellular Therapies for Multiple Myeloma: 2018 Update.Am Soc Clin Oncol Educ Book. 2018 May 23;38:e6-e15. doi: 10.1200/EDBK_200889. Am Soc Clin Oncol Educ Book. 2018. PMID: 30231373 Review.
-
Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen.Front Immunol. 2019 Jul 16;10:1613. doi: 10.3389/fimmu.2019.01613. eCollection 2019. Front Immunol. 2019. PMID: 31379824 Free PMC article. Review.
Cited by
-
Advances in adoptive cellular immunotherapy and therapeutic breakthroughs in multiple myeloma.Exp Hematol Oncol. 2024 Oct 28;13(1):105. doi: 10.1186/s40164-024-00576-6. Exp Hematol Oncol. 2024. PMID: 39468695 Free PMC article. Review.
-
CAR T-cell therapy in cancer: Integrating nursing perspectives for enhanced patient care.Asia Pac J Oncol Nurs. 2024 Aug 23;11(10):100579. doi: 10.1016/j.apjon.2024.100579. eCollection 2024 Oct. Asia Pac J Oncol Nurs. 2024. PMID: 39315365 Free PMC article. Review.
-
Nurses' roles in CAR-T therapy for B-cell malignancies and managing associated cytokine release syndrome.Asia Pac J Oncol Nurs. 2023 Dec 28;11(2):100367. doi: 10.1016/j.apjon.2023.100367. eCollection 2024 Feb. Asia Pac J Oncol Nurs. 2023. PMID: 38304228 Free PMC article. Review.
-
BCMA-targeted therapies in multiple myeloma: advances, challenges and future prospects.Med Oncol. 2025 May 8;42(6):204. doi: 10.1007/s12032-025-02753-x. Med Oncol. 2025. PMID: 40338452 Review.
-
Emerging Concepts in Immuno-Oncology: Insights from Natural Language Processing-Driven Co-Occurrence Analysis.ACS Omega. 2025 Jun 27;10(27):28587-28614. doi: 10.1021/acsomega.5c00693. eCollection 2025 Jul 15. ACS Omega. 2025. PMID: 40686960 Free PMC article. Review.
References
-
- Brown R.D., Pope B., Murray A., Esdale W., Sze D.M., Gibson J., Ho P.J., Hart D., Joshua D. Dendritic Cells from Patients with Myeloma Are Numerically Normal but Functionally Defective as They Fail to Up-Regulate CD80 (B7-1) Expression after huCD40LT Stimulation Because of Inhibition by Transforming Growth Factor-Beta1 and Interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.V98.10.2992. - DOI - PubMed
-
- Kazmi S.M., Nusrat M., Gunaydin H., Cornelison A.M., Shah N., Kebriaei P., Nieto Y., Parmar S., Popat U.R., Oran B., et al. Outcomes among High-Risk and Standard-Risk Multiple Myeloma Patients Treated with High-Dose Chemotherapy and Autologous Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2015;15:687–693. doi: 10.1016/j.clml.2015.07.641. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

