Mucosal Immunization Has Benefits over Traditional Subcutaneous Immunization with Group A Streptococcus Antigens in a Pilot Study in a Mouse Model
- PMID: 38006056
- PMCID: PMC10674289
- DOI: 10.3390/vaccines11111724
Mucosal Immunization Has Benefits over Traditional Subcutaneous Immunization with Group A Streptococcus Antigens in a Pilot Study in a Mouse Model
Abstract
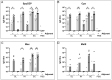
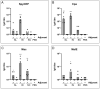
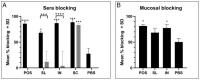
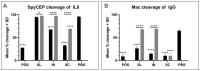
Group A Streptococcus (GAS) is a major human pathogen for which there is no licensed vaccine. To protect against infection, a strong systemic and mucosal immune response is likely to be necessary to prevent initial colonization and any events that might lead to invasive disease. A broad immune response will be necessary to target the varied GAS serotypes and disease presentations. To this end, we designed a representative panel of recombinant proteins to cover the stages of GAS infection and investigated whether mucosal and systemic immunity could be stimulated by these protein antigens. We immunized mice sublingually, intranasally and subcutaneously, then measured IgG and IgA antibody levels and functional activity through in vitro assays. Our results show that both sublingual and intranasal immunization in the presence of adjuvant induced both systemic IgG and mucosal IgA. Meanwhile, subcutaneous immunization generated only a serum IgG response. The antibodies mediated binding and killing of GAS cells and blocked binding of GAS to HaCaT cells, particularly following intranasal and subcutaneous immunizations. Further, antigen-specific assays revealed that immune sera inhibited cleavage of IL-8 by SpyCEP and IgG by Mac/IdeS. These results demonstrate that mucosal immunization can induce effective systemic and mucosal antibody responses. This finding warrants further investigation and optimization of humoral and cellular responses as a viable alternative to subcutaneous immunization for urgently needed GAS vaccines.
Keywords: Streptococcus pyogenes; group A Streptococcus; intranasal; mucosal; multicomponent; strep A; sublingual; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Multicomponent Vaccines against Group A Streptococcus Can Effectively Target Broad Disease Presentations.Vaccines (Basel). 2021 Sep 15;9(9):1025. doi: 10.3390/vaccines9091025. Vaccines (Basel). 2021. PMID: 34579262 Free PMC article.
-
A Multicomponent Vaccine Provides Immunity against Local and Systemic Infections by Group A Streptococcus across Serotypes.mBio. 2019 Nov 26;10(6):e02600-19. doi: 10.1128/mBio.02600-19. mBio. 2019. PMID: 31772056 Free PMC article.
-
Preparation and preclinical evaluation of experimental group B streptococcus type III polysaccharide-cholera toxin B subunit conjugate vaccine for intranasal immunization.Vaccine. 2000 Nov 22;19(7-8):850-61. doi: 10.1016/s0264-410x(00)00226-7. Vaccine. 2000. PMID: 11115709
-
Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice.Vaccine. 2011 Jun 24;29(29-30):4778-84. doi: 10.1016/j.vaccine.2011.04.083. Epub 2011 May 10. Vaccine. 2011. PMID: 21565244
-
The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines.Auris Nasus Larynx. 2022 Feb;49(1):1-10. doi: 10.1016/j.anl.2021.07.003. Epub 2021 Jul 23. Auris Nasus Larynx. 2022. PMID: 34304944 Review.
Cited by
-
Thymic stromal lymphopoietin improves protective immunity of the SARS-CoV-2 subunit vaccine by inducing dendritic cell-dependent germinal center response.J Virol. 2025 Apr 15;99(4):e0232324. doi: 10.1128/jvi.02323-24. Epub 2025 Mar 4. J Virol. 2025. PMID: 40035515 Free PMC article.
References
-
- Watkins D.A., Johnson C.O., Colquhoun S.M., Karthikeyan G., Beaton A., Bukhman G., Forouzanfar M.H., Longenecker C.T., Mayosi B.M., Mensah G.A., et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N. Engl. J. Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. - DOI - PubMed
-
- Bennett J., Zhang J., Leung W., Jack S., Oliver J., Webb R., Wilson N., Sika-Paotonu D., Harwood M., Baker M.G. Rising Ethnic Inequalities in Acute Rheumatic Fever and Rheumatic Heart Disease, New Zealand, 2000–2018. Emerg. Infect. Dis. 2021;27:36–46. doi: 10.3201/eid2701.191791. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

