Longitudinal Analysis of Neuraminidase and Hemagglutinin Antibodies to Influenza A Viruses after Immunization with Seasonal Inactivated Influenza Vaccines
- PMID: 38006063
- PMCID: PMC10675551
- DOI: 10.3390/vaccines11111731
Longitudinal Analysis of Neuraminidase and Hemagglutinin Antibodies to Influenza A Viruses after Immunization with Seasonal Inactivated Influenza Vaccines
Abstract
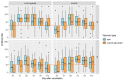
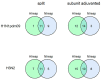
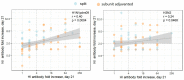
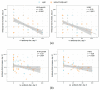
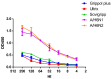
Neuraminidase (NA)-based immunity could reduce the harmful impact of novel antigenic variants of influenza viruses. The detection of neuraminidase-inhibiting (NI) antibodies in parallel with anti-hemagglutinin (HA) antibodies may enhance research on the immunogenicity and duration of antibody responses to influenza vaccines. To assess anti-NA antibodies after vaccination with seasonal inactivated influenza vaccines, we used the enzyme-linked lectin assay, and anti-HA antibodies were detected in the hemagglutination inhibition assay. The dynamics of the anti-NA antibody response differed depending on the virus subtype: antibodies to A/H3N2 virus neuraminidase increased later than antibodies to A/H1N1pdm09 subtype neuraminidase and persisted longer. In contrast to HA antibodies, the fold increase in antibody titers to NA after vaccination poorly depended on the preexisting level. At the same time, NA antibody levels after vaccination directly correlated with titers before vaccination. A difference was found in response to NA antigen between split and subunit-adjuvanted vaccines and in NA functional activity in the vaccine formulations.
Keywords: antibodies; influenza; neuraminidase-inhibition; persistence; vaccines.
Conflict of interest statement
The authors declare no financial interests/personal relationships related to this manuscript.
Figures

Similar articles
-
Neuraminidase Antibody Response to Homologous and Drifted Influenza A Viruses After Immunization with Seasonal Influenza Vaccines.Vaccines (Basel). 2024 Nov 27;12(12):1334. doi: 10.3390/vaccines12121334. Vaccines (Basel). 2024. PMID: 39771996 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Repeated Influenza Vaccination Boosts and Maintains H1N1pdm09 Neuraminidase Antibody Titers.Front Immunol. 2021 Oct 14;12:748264. doi: 10.3389/fimmu.2021.748264. eCollection 2021. Front Immunol. 2021. PMID: 34721417 Free PMC article.
-
Inactivated H7 Influenza Virus Vaccines Protect Mice despite Inducing Only Low Levels of Neutralizing Antibodies.J Virol. 2017 Sep 27;91(20):e01202-17. doi: 10.1128/JVI.01202-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768855 Free PMC article.
-
Contribution of Neuraminidase to the Efficacy of Seasonal Split Influenza Vaccines in the Ferret Model.J Virol. 2022 Mar 23;96(6):e0195921. doi: 10.1128/jvi.01959-21. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107371 Free PMC article.
Cited by
-
Occurrence of Circulating Antibodies against the Hemagglutinins of Influenza Viruses in the 2022/2023 Epidemic Season in Poland.Viruses. 2024 Jul 9;16(7):1105. doi: 10.3390/v16071105. Viruses. 2024. PMID: 39066267 Free PMC article.
-
The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence.Int J Mol Sci. 2025 Aug 2;26(15):7476. doi: 10.3390/ijms26157476. Int J Mol Sci. 2025. PMID: 40806604 Free PMC article.
-
Neuraminidase Antibody Response to Homologous and Drifted Influenza A Viruses After Immunization with Seasonal Influenza Vaccines.Vaccines (Basel). 2024 Nov 27;12(12):1334. doi: 10.3390/vaccines12121334. Vaccines (Basel). 2024. PMID: 39771996 Free PMC article.
-
Characterizing the Short- and Long-Term Temporal Dynamics of Antibody Responses to Influenza Vaccination.medRxiv [Preprint]. 2025 Feb 27:2025.02.26.25322965. doi: 10.1101/2025.02.26.25322965. medRxiv. 2025. PMID: 40061340 Free PMC article. Preprint.
-
Anti-neuraminidase immunity in the combat against influenza.Expert Rev Vaccines. 2024 Jan-Dec;23(1):474-484. doi: 10.1080/14760584.2024.2343689. Epub 2024 Apr 23. Expert Rev Vaccines. 2024. PMID: 38632930 Free PMC article. Review.
References
-
- Kon T.C., Onu A., Berbecila L., Lupulescu E., Ghiorgisor A., Kersten G.F., Cui Y.-Q., Amorij J.-P., Van der Pol L. Influenza vaccine manufacturing: Effect of inactivation, splitting and site of manufacturing. Comparison of influenza vaccine production processes. PLoS ONE. 2016;11:e0150700. doi: 10.1371/journal.pone.0150700. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

