Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment
- PMID: 38006112
- PMCID: PMC10674731
- DOI: 10.3390/polym15224388
Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment
Abstract
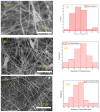
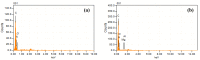
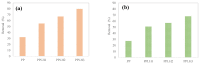
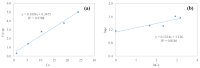
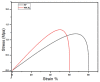
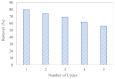
Layered double hydroxides (LDHs) with their unique structural chemistry create opportunities to be modified with polymers, making different nanocomposites. In the current research, a novel PET-PAN embedded with Mg-AI-LDH-PVA nanocomposite membrane was fabricated through electrospinning. SEM, EDX, FTIR, XRD, and AFM were carried out to investigate the structure and morphology of the nanocomposite membrane. The characterization of the optimized nanocomposite membrane showed a beadless, smooth structure with a nanofiber diameter of 695 nm. The water contact angle and tensile strength were 16° and 1.4 Mpa, respectively, showing an increase in the hydrophilicity and stability of the nanocomposite membrane by the addition of Mg-Al-LDH-PVA. To evaluate the adsorption performance of the nanocomposite membrane, operating parameters were achieved for Cr(VI) and methyl orange at pH 2.0 and pH 4.0, respectively, including contact time, adsorbate dose, and pollutant concentration. The adsorption data of the nanocomposite membrane showed the removal of 68% and 80% for Cr(VI) and methyl orange, respectively. The process of adsorption followed a Langmuir isotherm model that fit well and pseudo-2nd order kinetics with R2 values of 0.97 and 0.99, respectively. The recycling results showed the membrane's stability for up to five cycles. The developed membrane can be used for efficient removal of pollutants from wastewater.
Keywords: adsorption; electrospinning; layered double hydroxides; nanocomposite membrane; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









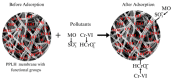
References
-
- Tariq M., Ali Baig S., Shams D.F., Hussain S., Hussain R., Qadir A., Maryam H.S., Khan Z.U., Sattar S., Xu X. Dye Wastewater Treatment Using Wheat Straw Biochar in Gadoon Industrial Areas of Swabi, Pakistan. Water Conserv. Sci. Eng. 2022;7:315–326.
-
- Baburaj M., Veeran M.G., Painuly D., Sreelekshmi S., Rajkumar R., Aprem A.S. Fabrication and characterisation of polycaprolactone/gelatin/chitosan (PCL/GEL/CHI) electrospun nano-membranes for wastewater purification. Desalination. 2023;563:116709.
-
- Wu S., Li K., Shi W., Cai J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022;210:76–84. - PubMed
-
- Alharbi H.F., Haddad M.Y., Aijaz M.O., Assaifan A.K., Karim M.R. Electrospun bilayer PAN/chitosan nanofiber membranes incorporated with metal oxide nanoparticles for heavy metal ion adsorption. Coatings. 2020;10:285. doi: 10.3390/coatings10030285. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

