Telocyte-Derived Exosomes Provide an Important Source of Wnts That Inhibits Fibrosis and Supports Regeneration and Repair of Endometrium
- PMID: 38006220
- PMCID: PMC10676634
- DOI: 10.1177/09636897231212746
Telocyte-Derived Exosomes Provide an Important Source of Wnts That Inhibits Fibrosis and Supports Regeneration and Repair of Endometrium
Abstract
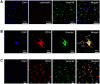
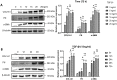
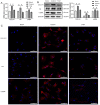
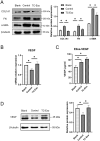
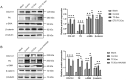
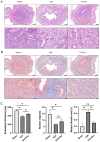
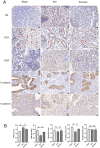
Intrauterine adhesions (IUAs) often occurred after common obstetrical and gynecological procedures or infections in women of reproductive age. It was characterized by the formation of endometrial fibrosis and prevention of endometrial regeneration, usually with devastating fertility consequences and poor treatment outcomes so far. Telocytes (TCs), as a novel interstitial cell type, present in female uterus with in vitro therapeutic potential in decidualization-defective gynecologic diseases. This study aims to further investigate the role of TC-derived Wnt ligands carried by exosomes (Exo) in reversal of fibrosis and enhancement of regeneration repair in endometrium. IUA cellular and animal models were established from endometrial stromal cells (ESCs) and mice, followed with treatment of TC-conditioned medium (TCM) or TC-derived Exo. In cellular model, fibrosis markers (collagen type 1 alpha 1 [COL1A1], fibronectin [FN], and α-smooth muscle actin [α-SMA]), angiogenesis (vascular endothelial growth factor [VEGF]), and pathway protein (β-catenin) were determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR), Western blotting (WB), and immunofluorescence. Results showed that, TCs (either TCM or TC-derived Exo) provide a source of Wnts that inhibit cellular fibrosis, as evidenced by significantly elevated VEGF and β-catenin with decreased fibrotic markers, whereas TCs lost salvage on fibrosis after being blocked with Wnt/β-catenin inhibitors (XAV939 or ETC-159). Further in mouse model, regeneration repair (endometrial thickness, number of glands, and fibrosis area ratio), fibrosis markers (fibronectin [FN]), mesenchymal-epithelial transition (MET) (E-cadherin, N-cadherin), and angiogenesis (VEGF, microvessel density [MVD]) were studied by hematoxylin-eosin (HE), Masson staining, and immunohistochemistry. Results demonstrated that TC-Exo treatment effectively promotes regeneration repair of endometrium by relieving fibrosis, enhancing MET, and angiogenesis. These results confirmed new evidence for therapeutic perspective of TC-derived Exo in IUAs.
Keywords: endometrial stromal cells (ESCs); exosome (Exo); fibrosis; intrauterine adhesions (IUAs); telocytes (TCs).
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome—one century later. Fertil Steril. 2008;89(4):759–79. - PubMed
-
- Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, Yauger BJ, Hill MJ. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215(3):267–75.e7. - PubMed
-
- Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal–epithelial transition in endometrial function. Hum Reprod Update. 2018;25(1):114–33. - PubMed
-
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

