Chicken manure application alters microbial community structure and the distribution of antibiotic-resistance genes in rhizosphere soil of Cinnamomum camphora forests
- PMID: 38006232
- PMCID: PMC10710299
- DOI: 10.1093/femsec/fiad155
Chicken manure application alters microbial community structure and the distribution of antibiotic-resistance genes in rhizosphere soil of Cinnamomum camphora forests
Abstract
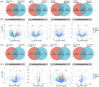
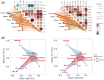
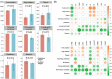
The distribution of antibiotic-resistance genes (ARGs) in environmental soil is greatly affected by livestock and poultry manure fertilization, the application of manure will lead to antibiotic residues and ARGs pollution, and increase the risk of environmental pollution and human health. Cinnamomum camphora is an economically significant tree species in Fujian Province, China. Here, through high-throughput sequencing analysis, significant differences in the composition of the bacterial community and ARGs were observed between fertilized and unfertilized rhizosphere soil. The application of chicken manure organic fertilizer significantly increased the relative abundance and alpha diversity of the bacterial community and ARGs. The content of organic matter, soluble organic nitrogen, available phosphorus, nitrate reductase, hydroxylamine reductase, urease, acid protease, β-glucosidase, oxytetracycline, and tetracycline in the soil of C. camphora forests have significant effects on bacterial community and ARGs. Significant correlations between environmental factors, bacterial communities, and ARGs were observed in the rhizosphere soil of C. camphora forests according to Mantel tests. Overall, the findings of this study revealed that chicken manure organic fertilizer application has a significant effect on the bacterial community and ARGs in the rhizosphere soil of C. camphora forests, and several environmental factors that affect the bacterial community and ARGs were identified.
Keywords: Cinnamomum camphora; antibiotic-resistance genes; bacterial community; chicken manure organic fertilizer; environmental factor.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Abbassi MS, Badi S, Lengliz Set al. Hiding in plain sight—wildlife as a neglected reservoir and pathway for the spread of antimicrobial resistance: a narrative review. FEMS Microbiol Ecol. 2022;98:fiac045. - PubMed
-
- Adebola AE, Ewulo BS, Arije DN. Effects of different animal manures on soil physical and microbial properties. Appl Trop Agric. 2017;22:128–33.
-
- Adubasim CV, Igwenagu CM, Josiah GOet al. Substitution of manure source and aerator in nursery media on sandy loam topsoil and their fertility indices 4 months after formulation. Int J Recyc Org Waste Agric. 2018;7:305–12.
-
- Allen HK, Donato J, Wang HHet al. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8:251–9. - PubMed
-
- Allison SD, Czimczik CI, Treseder KK. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Change Biol. 2008;14:1156–68.

