Nigrostriatal tau pathology in parkinsonism and Parkinson's disease
- PMID: 38006313
- PMCID: PMC10834249
- DOI: 10.1093/brain/awad388
Nigrostriatal tau pathology in parkinsonism and Parkinson's disease
Erratum in
-
Correction to: Nigrostriatal tau pathology in parkinsonism and Parkinson's disease.Brain. 2025 May 13;148(5):e50-e51. doi: 10.1093/brain/awaf146. Brain. 2025. PMID: 40298590 Free PMC article. No abstract available.
Abstract
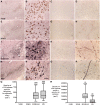
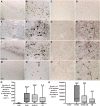
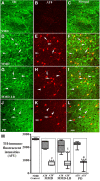
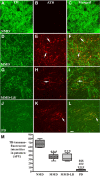
While Parkinson's disease remains clinically defined by cardinal motor symptoms resulting from nigrostriatal degeneration, it is now appreciated that the disease commonly consists of multiple pathologies, but it is unclear where these co-pathologies occur early in disease and whether they are responsible for the nigrostriatal degeneration. For the past number of years, we have been studying a well-characterized cohort of subjects with motor impairment that we have termed mild motor deficits. Motor deficits were determined on a modified and validated Unified Parkinson's Disease Rating Scale III but were insufficient in degree to diagnose Parkinson's disease. However, in our past studies, cases in this cohort had a selection bias, as both a clinical syndrome in between no motor deficits and Parkinson's disease, plus nigral Lewy pathology as defined post-mortem, were required for inclusion. Therefore, in the current study, we only based inclusion on the presence of a clinical phenotype with mild motor impairment insufficient to diagnose Parkinson's disease. Then, we divided this group further based upon whether or not subjects had a synucleinopathy in the nigrostriatal system. Here we demonstrate that loss of nigral dopaminergic neurons, loss of putamenal dopaminergic innervation and loss of the tyrosine hydroxylase-phenotype in the substantia nigra and putamen occur equally in mild motor deficit groups with and without nigral alpha-synuclein aggregates. Indeed, the common feature of these two groups is that both have similar degrees of AT8 positive phosphorylated tau, a pathology not seen in the nigrostriatal system of age-matched controls. These findings were confirmed with early (tau Ser208 phosphorylation) and late (tau Ser396/Ser404 phosphorylation) tau markers. This suggests that the initiation of nigrostriatal dopaminergic neurodegeneration occurs independently of alpha-synuclein aggregation and can be tau mediated.
Keywords: Parkinson’s disease; alpha-synuclein; dopaminergic neurodegeneration; parkinsonism; tau.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Are we entering the 'Tau-lemaic' era of Parkinson's disease?Brain. 2024 Feb 1;147(2):330-332. doi: 10.1093/brain/awae002. Brain. 2024. PMID: 38190424 No abstract available.
Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes.Brain. 2019 Nov 1;142(11):3565-3579. doi: 10.1093/brain/awz296. Brain. 2019. PMID: 31580415 Free PMC article.
-
CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson's disease.Brain. 2020 Dec 1;143(12):3717-3733. doi: 10.1093/brain/awaa269. Brain. 2020. PMID: 33118032
-
A Combined α-Synuclein/Fibril (SynFib) Model of Parkinson-Like Synucleinopathy Targeting the Nigrostriatal Dopamine System.J Parkinsons Dis. 2022;12(8):2307-2320. doi: 10.3233/JPD-223452. J Parkinsons Dis. 2022. PMID: 36189605 Free PMC article. Review.
-
A Cortical Pathogenic Theory of Parkinson's Disease.Neuron. 2018 Sep 19;99(6):1116-1128. doi: 10.1016/j.neuron.2018.07.028. Neuron. 2018. PMID: 30236282 Review.
Cited by
-
Synaptic sabotage: How Tau and α-Synuclein undermine synaptic health.J Cell Biol. 2025 Feb 3;224(2):e202409104. doi: 10.1083/jcb.202409104. Epub 2024 Dec 24. J Cell Biol. 2025. PMID: 39718548 Review.
-
Additive effect of distant Lewy bodies and tau seeds injections on nigral degeneration in macaques.NPJ Parkinsons Dis. 2025 Apr 13;11(1):75. doi: 10.1038/s41531-025-00938-9. NPJ Parkinsons Dis. 2025. PMID: 40221462 Free PMC article.
-
Protein regulatory network mediated by palmitoylation modifications in the pathological progression of Parkinson's disease: a narrative review.Front Immunol. 2025 Jul 9;16:1615001. doi: 10.3389/fimmu.2025.1615001. eCollection 2025. Front Immunol. 2025. PMID: 40703511 Free PMC article. Review.
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
Parkinsonism in Alzheimer's disease without Lewy bodies in association with nigral neuron loss: A data-driven clinicopathologic study.Alzheimers Dement. 2025 Mar;21(3):e14628. doi: 10.1002/alz.14628. Alzheimers Dement. 2025. PMID: 40042515 Free PMC article.
References
-
- Dickson DW, Braak H, Duda JE, et al. . Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. - PubMed
-
- Gibb WRG, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology. 1988;38:1402–1402. - PubMed
-
- Braak H, del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

