Characteristics of post hoc subgroup analyses of oncology clinical trials: a systematic review
- PMID: 38006333
- PMCID: PMC11025370
- DOI: 10.1093/jncics/pkad100
Characteristics of post hoc subgroup analyses of oncology clinical trials: a systematic review
Abstract
Background: Subgroup analyses in clinical trials assess intervention effects on specific patient subgroups, ensuring generalizability. However, they are usually only able to generate hypotheses rather than definitive conclusions. This study examined the prevalence and characteristics of post hoc subgroup analysis in oncology.
Methods: We systematically reviewed published subgroup analyses from 2000 to 2022. We included articles presenting secondary, post hoc, or subgroup analyses of interventional clinical trials in oncology, cancer survivorship, or cancer screening, published separately from the original clinical trial publication. We collected cancer type, year of publication, where and how subgroup analyses were reported, and funding.
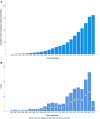
Results: Out of 16 487 screened publications, 1612 studies were included, primarily subgroup analyses of treatment trials for solid tumors (82%). Medical writers contributed to 31% of articles, and 58% of articles reported conflicts of interest. Subgroup analyses increased significantly over time, with 695 published between 2019 and 2022, compared to 384 from 2000 to 2014. Gastrointestinal tumors (25%) and lymphoid lineage tumors (39%) were the most frequently studied solid and hematological malignancies, respectively. Industry funding and reporting of conflicts of interest increased over time. Subgroup analyses often neglected to indicate their secondary nature in the title. Most authors were from high-income countries, most commonly North America (45%).
Conclusions: This study demonstrates the rapidly growing use of post hoc subgroup analysis of oncology clinical trials, revealing that the majority are supported by pharmaceutical companies, and they frequently fail to indicate their secondary nature in the title. Given the known methodological limitations of subgroup analyses, caution is recommended among authors, readers, and reviewers when conducting and interpreting these studies.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Medical writers were involved in the preparation of 31% of the articles, and COI was reported in 58%. The remaining 44% either did not report COI or reported no COI. The most recent period, 2019-2022, had a considerably higher proportion of articles reporting any kind of funding compared to other periods (
Interventional drug trials reported the highest percentages of funding, pharmaceutical company funding, COIs, and usage of medical writers compared to other types of trials (
J.A., M.A., M.A., N.O., Y.H., H.M., G.R.M., A.H.K, A.M.G, D.A.R. H.M., and D.B. reported no conflict of interests. R.C. reported consulting for Janssen, Sanofi Pasteur, and Adaptive Biotechnologies. S.A. received honoraria from Jansen and Sanofi. E.R.S.C receives research funding from Arnold Ventures.
Figures


References
-
- Guyatt G, Rennie D, Meade MO, Cook D. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed. New York, NY: McGraw-Hill; 2015:507-514.
-
- Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1630-1642. doi:10.1016/S1470-2045(20)30525-8 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
