Alcaligenes lipid A functions as a superior mucosal adjuvant to monophosphoryl lipid A via the recruitment and activation of CD11b+ dendritic cells in nasal tissue
- PMID: 38006376
- PMCID: PMC10823578
- DOI: 10.1093/intimm/dxad045
Alcaligenes lipid A functions as a superior mucosal adjuvant to monophosphoryl lipid A via the recruitment and activation of CD11b+ dendritic cells in nasal tissue
Abstract
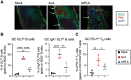
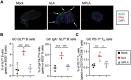
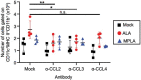
We previously demonstrated that Alcaligenes-derived lipid A (ALA), which is produced from an intestinal lymphoid tissue-resident commensal bacterium, is an effective adjuvant for inducing antigen-specific immune responses. To understand the immunologic characteristics of ALA as a vaccine adjuvant, we here compared the adjuvant activity of ALA with that of a licensed adjuvant (monophosphoryl lipid A, MPLA) in mice. Although the adjuvant activity of ALA was only slightly greater than that of MPLA for subcutaneous immunization, ALA induced significantly greater IgA antibody production than did MPLA during nasal immunization. Regarding the underlying mechanism, ALA increased and activated CD11b+ CD103- CD11c+ dendritic cells in the nasal tissue by stimulating chemokine responses. These findings revealed the superiority of ALA as a mucosal adjuvant due to the unique immunologic functions of ALA in nasal tissue.
Keywords: Alcaligenes; MPLA; chemokines; lipid A; nasal vaccine; stromal cells.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

