Assessment of hypoxia and oxidative-related changes in a lung-derived brain metastasis model by [64Cu][Cu(ATSM)] PET and proteomic studies
- PMID: 38006431
- PMCID: PMC10676347
- DOI: 10.1186/s13550-023-01052-8
Assessment of hypoxia and oxidative-related changes in a lung-derived brain metastasis model by [64Cu][Cu(ATSM)] PET and proteomic studies
Abstract
Background: Brain metastases (BM) are the most frequent malignant brain tumors. The aim of this study was to characterize the tumor microenvironment (TME) of BM and particularly hypoxia and redox state, known to play a role in tumor growth and treatment resistance with multimodal PET and MRI imaging, immunohistochemical and proteomic approaches in a human lung cancer (H2030-BrM3)-derived BM model in rats.
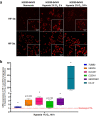
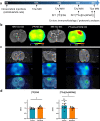
Results: First, in vitro studies confirmed that H2030-BrM3 cells respond to hypoxia with increasing expression of HIF-1, HIF-2 and their target genes. Proteomic analyses revealed, among expression changes, proteins associated with metabolism, oxidative stress, metal response and hypoxia signaling in particular in cortical BM. [64Cu][Cu(ATSM)] PET revealed a significant uptake by cortical BM (p < 0.01), while no uptake is observed in striatal BM 23 days after tumor implantation. Pimonidazole, HIF-1α, HIF-2α, CA-IX as well as GFAP, CTR1 and DMT1 immunostainings are positive in both BM.
Conclusion: Overall, [64Cu][Cu(ATSM)] imaging and proteomic results showed the presence of hypoxia and protein expression changes linked to hypoxia and oxidative stress in BM, which are more pronounced in cortical BM compared to striatal BM. Moreover, it emphasized the interest of [64Cu][Cu(ATSM)] PET to characterize TME of BM and depict inter-metastasis heterogeneity that could be useful to guide treatments.
Keywords: Brain metastasis; Copper-64; Cu-ATSM; HIF; Hypoxia; Imaging; Lung cancer; Oxidative stress; PET; Preclinical; Proteomic; Rodent.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
PET/MRI of Hypoxic Atherosclerosis Using 64Cu-ATSM in a Rabbit Model.J Nucl Med. 2016 Dec;57(12):2006-2011. doi: 10.2967/jnumed.116.172544. Epub 2016 Jul 7. J Nucl Med. 2016. PMID: 27390157 Free PMC article.
-
Application of 62Cu-diacetyl-bis (N4-methylthiosemicarbazone) PET imaging to predict highly malignant tumor grades and hypoxia-inducible factor-1α expression in patients with glioma.AJNR Am J Neuroradiol. 2013 Jan;34(1):92-9. doi: 10.3174/ajnr.A3159. Epub 2012 Jun 14. AJNR Am J Neuroradiol. 2013. PMID: 22700754 Free PMC article.
-
Multimodal evaluation of hypoxia in brain metastases of lung cancer and interest of hypoxia image-guided radiotherapy.Sci Rep. 2021 May 27;11(1):11239. doi: 10.1038/s41598-021-90662-0. Sci Rep. 2021. PMID: 34045576 Free PMC article.
-
Hypoxia imaging and theranostic potential of [64Cu][Cu(ATSM)] and ionic Cu(II) salts: a review of current evidence and discussion of the retention mechanisms.EJNMMI Res. 2020 Apr 9;10(1):33. doi: 10.1186/s13550-020-00621-5. EJNMMI Res. 2020. PMID: 32274601 Free PMC article. Review.
-
Focus on the Controversial Aspects of (64)Cu-ATSM in Tumoral Hypoxia Mapping by PET Imaging.Front Med (Lausanne). 2015 Aug 24;2:58. doi: 10.3389/fmed.2015.00058. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26380261 Free PMC article. Review.
Cited by
-
Hyperbaric oxygen therapy as an adjunt treatment for glioma and brain metastasis: a literature review.Med Gas Res. 2025 Sep 1;15(3):420-426. doi: 10.4103/mgr.MEDGASRES-D-24-00096. Epub 2025 Feb 7. Med Gas Res. 2025. PMID: 39923138 Free PMC article. Review.
-
Reproductive aging weakens offspring survival and constrains the telomerase response to herpesvirus in Pacific oysters.Sci Adv. 2024 Sep 13;10(37):eadq2311. doi: 10.1126/sciadv.adq2311. Epub 2024 Sep 11. Sci Adv. 2024. PMID: 39259784 Free PMC article.
-
Genomic and immune profiling of breast cancer brain metastases.Acta Neuropathol Commun. 2025 May 12;13(1):99. doi: 10.1186/s40478-025-02001-3. Acta Neuropathol Commun. 2025. PMID: 40355907 Free PMC article.
-
Metastatic brain tumors: from development to cutting-edge treatment.MedComm (2020). 2024 Dec 20;6(1):e70020. doi: 10.1002/mco2.70020. eCollection 2025 Jan. MedComm (2020). 2024. PMID: 39712454 Free PMC article. Review.
-
Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood-Brain Barrier and Blood-Tumor Barrier.Int J Mol Sci. 2025 Jul 22;26(15):7050. doi: 10.3390/ijms26157050. Int J Mol Sci. 2025. PMID: 40806198 Free PMC article. Review.
References
-
- Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773. doi: 10.1200/JCO.20.01255. - DOI - PMC - PubMed
-
- Ryken TC, Kuo JS, Prabhu RS, Sherman JH, Kalkanis SN, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of steroids in the treatment of adults with metastatic brain tumors. Clin Neurosurg. 2019;84:E189–E191. doi: 10.1093/neuros/nyy546. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

