3D printing injectable microbeads using a composite liposomal ink for local treatment of peritoneal diseases
- PMID: 38006449
- PMCID: PMC11052830
- DOI: 10.1007/s13346-023-01472-y
3D printing injectable microbeads using a composite liposomal ink for local treatment of peritoneal diseases
Abstract
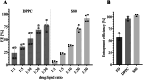
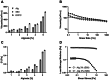
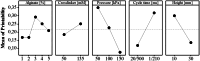
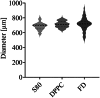
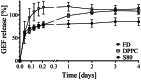
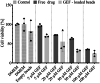
The peritoneal cavity offers an attractive administration route for challenging-to-treat diseases, such as peritoneal carcinomatosis, post-surgical adhesions, and peritoneal fibrosis. Achieving a uniform and prolonged drug distribution throughout the entire peritoneal space, though, is difficult due to high clearance rates, among others. To address such an unmet clinical need, alternative drug delivery approaches providing sustained drug release, reduced clearance rates, and a patient-centric strategy are required. Here, we describe the development of a 3D-printed composite platform for the sustained release of the tyrosine kinase inhibitor gefitinib (GEF), a small molecule drug with therapeutic applications for peritoneal metastasis and post-surgical adhesions. We present a robust method for the production of biodegradable liposome-loaded hydrogel microbeads that can overcome the pharmacokinetic limitations of small molecules with fast clearance rates, a current bottleneck for the intraperitoneal (IP) administration of these therapeutics. By means of an electromagnetic droplet printhead, we 3D printed microbeads employing an alginate-based ink loaded with GEF-containing multilamellar vesicles (MLVs). The sustained release of GEF from microbeads was demonstrated. In vitro studies on an immortalized human hepatic cancer cell line (Huh-7) proved concentration-dependent cell death. These findings demonstrate the potential of 3D-printed alginate microbeads containing liposomes for delivering small drug compounds into the peritoneum, overcoming previous limitations of IP drug delivery.
Keywords: 3D printing; Drop-on-demand manufacturing; Hydrogel microbeads; Liposomes; Peritoneal drug delivery; Sustained drug release.
© 2023. The Author(s).
Conflict of interest statement
No private study sponsors had any involvement in the study design, data collection, or interpretation of data presented in this manuscript. P.L. declares the following competing interests: she has consulted and received research grants from Lipoid, Sanofi-Aventis Deutschland and DSM Nutritional Products Ltd. R.E., A.G., S.A. and A.S. declare no competing interests.
Figures


References
-
- Braet H, Fransen P-P, Mariën R, Lollo G, Ceelen W, Vervaet C, Balcaen L, Vanhaecke F, Vanhove C, van der Vegte S, Gasthuys E, Vermeulen A, Dankers PYW, De Smedt SC, Remaut K. CO2-driven nebulization of pH-sensitive supramolecular polymers for intraperitoneal hydrogel formation and the treatment of peritoneal metastasis. ACS Appl Mater Interfaces. 2023;15:49022–49034. doi: 10.1021/acsami.3c11274. - DOI - PubMed

