Degradation studies of bisphenol S by ultrasound activated persulfate in aqueous medium
- PMID: 38006821
- PMCID: PMC10767634
- DOI: 10.1016/j.ultsonch.2023.106700
Degradation studies of bisphenol S by ultrasound activated persulfate in aqueous medium
Abstract
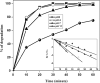
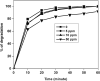
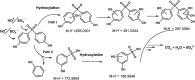
The degradation of recalcitrant organic pollutants by sulphate radical (SO4•-) represents one of the most recent developments in oxidation-based water treatment. In most cases, persulfate (PS) acts as a precursor of sulphate radicals. This study employed ultrasound-activated PS to generate reactive species, facilitating the degradation of bisphenol S (BPS), a well-known contaminant of emerging concern (CECs). An ultrasound with a frequency of 620 kHz and 80 W power was utilised for the degradation studies. The applied oxidation system successfully resulted in the complete degradation of BPS in both pure and real environmental water samples. Additionally, the Chemical oxygen demand (COD) was reduced to an acceptable limit in both matrices, with a reduction of 85 % in pure water and 73 % in river water. The degradation was monitored by varying chemical parameters such as pH, inorganic ions, and organics concentration. The results indicate that under specific pH conditions, the degradation efficiency followed the order of pH 3 > 4 > 7 > 11. The presence of coexisting matrices suppressed the efficiency by scavenging the reactive species. Utilizing high-resolution mass spectrometry (HRMS) analysis, this study identified seven intermediate products during identified during the degradation of BPS. Furthermore, a comprehensive mechanism has been deduced for the transformation and degradation process. All the results presented in this study underscore the applicability of the US/PS system in the removal of CECs.
Keywords: Advanced oxidation processes; Bisphenol S; Cavitation; Chemical Oxygen Demand; Persulfate.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Viglino L., Aboulfadl K., Prévost M., Sauvé S. Analysis of natural and synthetic estrogenic endocrine disruptors in environmental waters using online preconcentration coupled with LC-APPI-MS/MS. Talanta. 2008;76:1088–1096. - PubMed
-
- Liu J., Zhang L., Lu G., Jiang R., Yan Z., Li Y. Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment – a review. Ecotoxicol. Environ. Saf. 2021;208 - PubMed
-
- Nejumal K.K., Dineep D., Mohan M., Krishnan K.P., Aravind U.K., Aravindakumar C.T. Presence of bisphenol S and surfactants in the sediments of Kongsfjorden: a negative impact of human activities in Arctic? Environ. Monit. Assess. 2017;190:22. - PubMed
-
- Ji K., Hong S., Kho Y., Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Tech. 2013;47:8793–8800. - PubMed
-
- Moreman J., Lee O., Trznadel M., David A., Kudoh T., Tyler C.R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Tech. 2017;51:12796–12805. - PubMed
LinkOut - more resources
Full Text Sources

