Oxidative stress in the eye and its role in the pathophysiology of ocular diseases
- PMID: 38006824
- PMCID: PMC10701459
- DOI: 10.1016/j.redox.2023.102967
Oxidative stress in the eye and its role in the pathophysiology of ocular diseases
Abstract
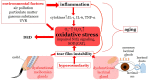
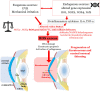
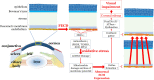
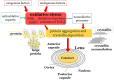
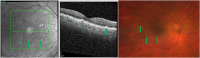
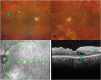
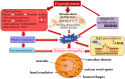
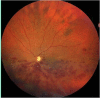
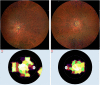
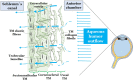
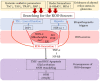
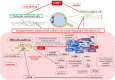
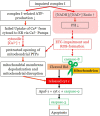
Oxidative stress occurs through an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense mechanisms of cells. The eye is particularly exposed to oxidative stress because of its permanent exposure to light and due to several structures having high metabolic activities. The anterior part of the eye is highly exposed to ultraviolet (UV) radiation and possesses a complex antioxidant defense system to protect the retina from UV radiation. The posterior part of the eye exhibits high metabolic rates and oxygen consumption leading subsequently to a high production rate of ROS. Furthermore, inflammation, aging, genetic factors, and environmental pollution, are all elements promoting ROS generation and impairing antioxidant defense mechanisms and thereby representing risk factors leading to oxidative stress. An abnormal redox status was shown to be involved in the pathophysiology of various ocular diseases in the anterior and posterior segment of the eye. In this review, we aim to summarize the mechanisms of oxidative stress in ocular diseases to provide an updated understanding on the pathogenesis of common diseases affecting the ocular surface, the lens, the retina, and the optic nerve. Moreover, we discuss potential therapeutic approaches aimed at reducing oxidative stress in this context.
Keywords: Antioxidants; Eye; Ocular diseases; Oxidative stress; Pathophysiology; Reactive oxygen species.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they do not have financial or other conflicts of interest. All authors made substantial contributions to the conception and design of the article, drafting or revising the article for critically important intellectual content and approved the final version of the article.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

