The NYCKidSeq randomized controlled trial: Impact of GUÍA digitally enhanced genetic results disclosure in diverse families
- PMID: 38006881
- PMCID: PMC10716481
- DOI: 10.1016/j.ajhg.2023.10.016
The NYCKidSeq randomized controlled trial: Impact of GUÍA digitally enhanced genetic results disclosure in diverse families
Abstract
Digital solutions are needed to support rapid increases in the application of genetic/genomic tests (GTs) in diverse clinical settings and patient populations. We developed GUÍA, a bilingual digital application that facilitates disclosure of GT results. The NYCKidSeq randomized controlled trial enrolled diverse children with neurologic, cardiac, and immunologic conditions who underwent GTs. The trial evaluated GUÍA's impact on understanding the GT results by randomizing families to results disclosure genetic counseling with GUÍA (intervention) or standard of care (SOC). Parents/legal guardians (participants) completed surveys at baseline, post-results disclosure, and 6 months later. Survey measures assessed the primary study outcomes of participants' perceived understanding of and confidence in explaining their child's GT results and the secondary outcome of objective understanding. The analysis included 551 diverse participants, 270 in the GUÍA arm and 281 in SOC. Participants in the GUÍA arm had significantly higher perceived understanding post-results (OR = 2.8, CI[1.004, 7.617], p = 0.049) and maintained higher objective understanding over time (OR = 1.1, CI[1.004, 1.127], p = 0.038) compared to SOC. There was no impact on perceived confidence. Hispanic/Latino(a) individuals in the GUÍA arm maintained higher perceived understanding (OR = 3.9, CI[1.603, 9.254], p = 0.003), confidence (OR = 2.7, CI[1.021, 7.277], p = 0.046), and objective understanding (OR = 1.1, CI[1.009, 1.212], p = 0.032) compared to SOC. This trial demonstrates that GUÍA positively impacts understanding of GT results in diverse parents of children with suspected genetic conditions and builds a case for utilizing GUÍA to deliver complex results. Continued development and evaluation of digital applications in diverse populations are critical for equitably scaling GT offerings in specialty clinics.
Keywords: digital solutions; digital tools; diverse populations; genetic counseling; genetic testing; genome sequencing; genomic medicine.
Copyright © 2023 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.E.K. has received speaker honoraria from Illumina, 23&Me, Allelica, and Regeneron Pharmaceuticals; received research funding from Allelica; and serves as a scientific advisory board member for Encompass Biosciences, Foresite Labs, and Galateo Bio. N.S.A.-H. is an equity holder of 23andMe; serves as a scientific advisory board member for Allelica; received personal fees from Genentech, Allelica, and 23andMe; received research funding from Akcea; and was previously employed by Regeneron Pharmaceuticals.
Figures



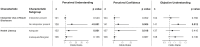
Update of
-
The NYCKidSeq randomized controlled trial: Impact of GUÍA digitally enhanced genetic counseling in racially and ethnically diverse families.medRxiv [Preprint]. 2023 Jul 7:2023.07.05.23292193. doi: 10.1101/2023.07.05.23292193. medRxiv. 2023. Update in: Am J Hum Genet. 2023 Dec 7;110(12):2029-2041. doi: 10.1016/j.ajhg.2023.10.016. PMID: 37461450 Free PMC article. Updated. Preprint.
Similar articles
-
Investigating the Impact of Screen-Sharing Visual Aids during Genomic Results Disclosure via Telehealth in Diverse Families in the TeleKidSeq Pilot Study.Public Health Genomics. 2025;28(1):85-101. doi: 10.1159/000542444. Epub 2025 Jan 17. Public Health Genomics. 2025. PMID: 39827879 Clinical Trial.
-
Evaluating parental personal utility of pediatric genetic and genomic testing in a diverse, multilingual population.HGG Adv. 2024 Jul 18;5(3):100321. doi: 10.1016/j.xhgg.2024.100321. Epub 2024 Jun 24. HGG Adv. 2024. PMID: 38918948 Free PMC article.
-
The NYCKidSeq randomized controlled trial: Impact of GUÍA digitally enhanced genetic counseling in racially and ethnically diverse families.medRxiv [Preprint]. 2023 Jul 7:2023.07.05.23292193. doi: 10.1101/2023.07.05.23292193. medRxiv. 2023. Update in: Am J Hum Genet. 2023 Dec 7;110(12):2029-2041. doi: 10.1016/j.ajhg.2023.10.016. PMID: 37461450 Free PMC article. Updated. Preprint.
-
GUÍA: a digital platform to facilitate result disclosure in genetic counseling.Genet Med. 2021 May;23(5):942-949. doi: 10.1038/s41436-020-01063-z. Epub 2021 Feb 2. Genet Med. 2021. PMID: 33531665 Free PMC article. Clinical Trial.
-
The NYCKidSeq project: study protocol for a randomized controlled trial incorporating genomics into the clinical care of diverse New York City children.Trials. 2021 Jan 14;22(1):56. doi: 10.1186/s13063-020-04953-4. Trials. 2021. PMID: 33446240 Free PMC article.
Cited by
-
Investigating the Impact of Screen-Sharing Visual Aids during Genomic Results Disclosure via Telehealth in Diverse Families in the TeleKidSeq Pilot Study.Public Health Genomics. 2025;28(1):85-101. doi: 10.1159/000542444. Epub 2025 Jan 17. Public Health Genomics. 2025. PMID: 39827879 Clinical Trial.
-
Employing effective recruitment and retention strategies to engage a diverse pediatric population in genomics research.Am J Hum Genet. 2024 Dec 5;111(12):2607-2617. doi: 10.1016/j.ajhg.2024.10.015. Epub 2024 Nov 19. Am J Hum Genet. 2024. PMID: 39566494 Free PMC article.
-
Evaluating parental personal utility of pediatric genetic and genomic testing in a diverse, multilingual population.HGG Adv. 2024 Jul 18;5(3):100321. doi: 10.1016/j.xhgg.2024.100321. Epub 2024 Jun 24. HGG Adv. 2024. PMID: 38918948 Free PMC article.
References
-
- Linn A.J., van Dijk L., Smit E.G., Jansen J., van Weert J.C.M. May you never forget what is worth remembering: the relation between recall of medical information and medication adherence in patients with inflammatory bowel disease. J. Crohns Colitis. 2013;7:e543–e550. - PubMed
-
- Dimatteo M.R. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ. Couns. 2004;55:339–344. - PubMed
-
- Patel H.V., Henrikson N.B., Ralston J.D., Leppig K., Scrol A., Jarvik G.P., DeVange S., Larson E.B., Hartzler A.L. Implementation matters: How patient experiences differ when genetic counseling accompanies the return of genetic variants of uncertain significance. AMIA Annu. Symp. Proc. 2021;2021:950–958. - PMC - PubMed
-
- Hoskovec J.M., Bennett R.L., Carey M.E., DaVanzo J.E., Dougherty M., Hahn S.E., LeRoy B.S., O’Neal S., Richardson J.G., Wicklund C.A. Projecting the Supply and Demand for Certified Genetic Counselors: a Workforce Study. J. Genet. Couns. 2018;27:16–20. - PubMed
-
- Gordon E.S., Babu D., Laney D.A. The future is now: Technology’s impact on the practice of genetic counseling. Am. J. Med. Genet. C Semin. Med. Genet. 2018;178:15–23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

